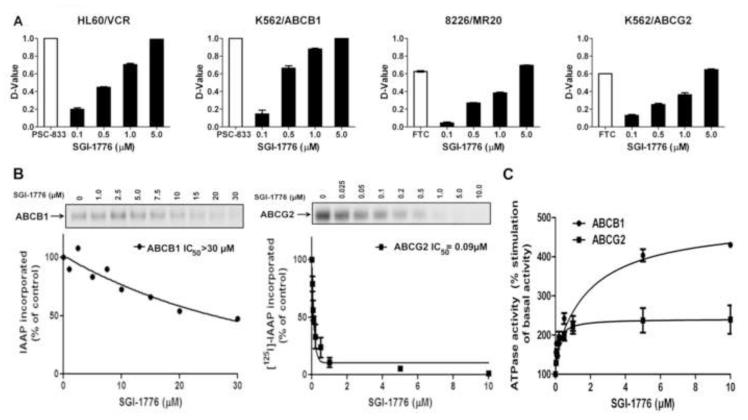
Figure 7.
A. SGI-1776 increases substrate uptake in cells expressing ABCB1 or ABCG2. 1 × 106 HL60/VCR and K562/ABCB1 cells, expressing ABCB1, and 8226/MR20 and K562/ABCG2 cells, expressing ABCG2, were exposed to their respective fluorescent substrates DiOC2(3) and pheophorbide A (PhA) in the presence of SGI-1776 in increasing concentrations or the established ABCB1 modulator PSC-833 or ABCG2 modulator fumitremorgin C (FTC) for 30 minutes at 37°C. Cellular fluorescence was measured by flow cytometry, and compared by D-values. Means ± SEM. of triplicate treatments are shown. B. SGI-1776 decreases [125I]-IAAP photolabeling of ABCB1 and ABCG2. Crude membranes containing ABCB1 or ABCG2 were incubated with 0-30 μM SGI-1776 for 5 minutes, then 3-6 nM [125I]-IAAP (2200 Ci/mmole) for five additional minutes. IAAP labeling of ABCB1 or ABCG2 was then measured as described in Materials and Methods and plotted against SGI-1776 concentration. Means ± SD from three independent experiments are shown. C. SGI-1776 increases ABCB1 and ABCG2 ATPase activity. Crude membrane preparations containing ABCB1 or ABCG2 were incubated with SGI-1776 in increasing concentrations in the presence or absence of beryllium sulfate and sodium fluoride (BeFx) or sodium orthovanadate, respectively. The amount of inorganic phosphate released and the vanadate or BeFx-sensitive ATPase activity were measured as described in Materials and Methods. The average from independent duplicate experiments is shown, with error bars representing standard error.