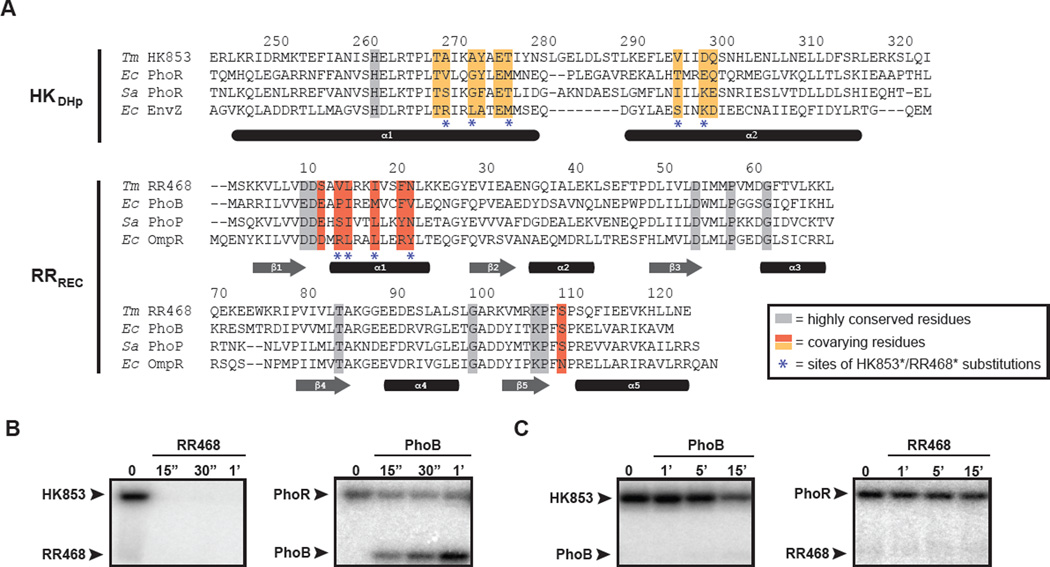
Figure 1. Specificity residues in two-component signaling proteins.
(A) Multiple sequence alignment of histidine kinases (DHp domain only) and response regulator (receiver domain only), with specificity residues and highly conserved residues highlighted. Species abbreviations: (Ec) Escherichia coli; (Sa) Staphylococcus aureus; (Tm) Thermotoga maritima. Interacting partners are arranged in the same order in both HK and RR alignments. Sequences are numbered according to the Tm proteins, with the last digit of each number positioned above the relevant amino acid residue. (B) HK853 and (C) PhoR phosphotransfer specificity. Each histidine kinase construct was autophosphorylated with [32P-γ]ATP and then incubated with the response regulator indicated at room temperature. Samples were taken at the time points indicated and phosphotransfer assessed by SDS-PAGE and phosphorimaging. Arrowheads indicate the position of autophosphorylated kinase or phosphorylated response regulator. Also see Figure S1.