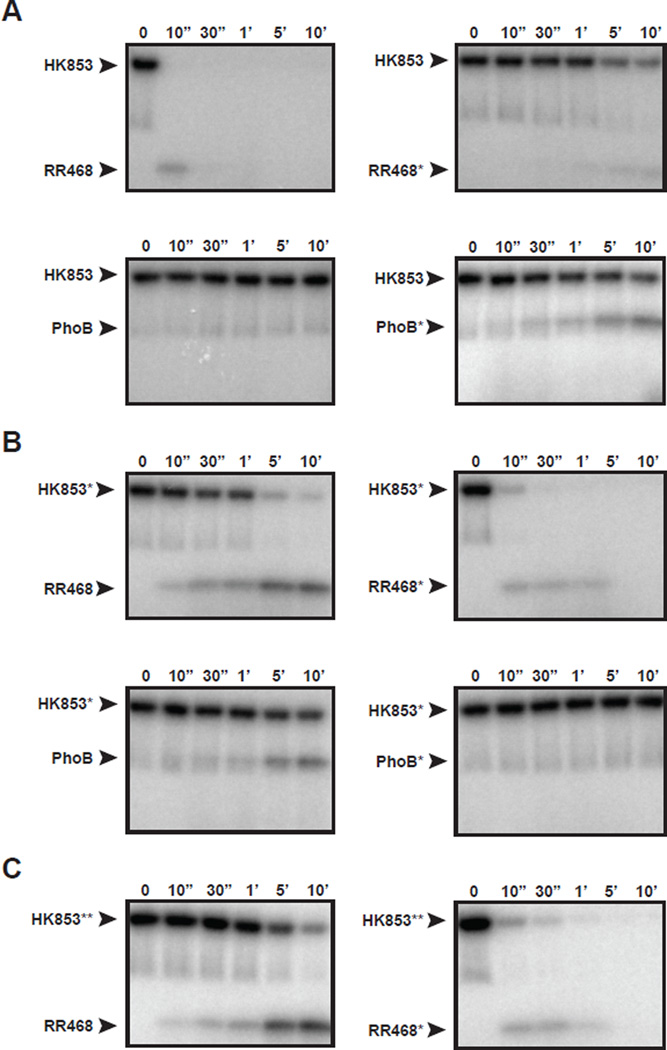
Figure 2. Rational rewiring of phosphotransfer specificity.
Phosphotransfer assays for wild-type and mutant two-component proteins. In each panel, the histidine kinase indicated was autophosphorylated with [32P-γ]ATP and then incubated with the response regulator indicated at 4 °C. Samples were taken at the time points indicated and phosphotransfer assessed by SDS-PAGE and phosphorimaging. (A) Wild-type HK853 and (B) HK853* (which harbors the substitutions A268V, A271G, T275M, V294T, D297E) were tested for phosphotransfer to RR468, RR468*, PhoB, and PhoB*. RR468* contains the substitutions V13P, L14I, I17M, and N21V. PhoB* contains the substitutions P13V, I14L, M17I, and V21N. (C) HK853**, which harbors the substitutions A268V, A271G, and T275M was tested for phosphotransfer to RR468 and RR468*. Also see Figures S2–S3.