Abstract
CONTEXT:
Hospitalized patients with acute exacerbations of chronic obstructive pulmonary disease (AECOPD) may be managed by either respiratory specialists (RS) or general medicine physicians (GMP). While previous studies have audited the hospital AECOPD management of RS, only a small number of studies have evaluated the management of GMP.
AIMS:
The aims of this study were to firstly examine the differences in AECOPD management of GMP and RS and secondly compare their care to national COPD guidelines.
METHODS:
A retrospective review was undertaken of consecutive AECOPD patients admitted to two hospitals (one hospital where all AECOPD patients were managed by RS and another where all AECOPD patients were managed by GMP) over a 3-month period. Electronic medical records, medical case notes, pathology and radiology data for the admission were reviewed.
RESULTS:
There were 201 COPD exacerbations in 169 patients (49.7% male, mean age 72.3). GMP managed 84 (41.7%) exacerbations. In comparison to RS, GMP performed fewer spirometry tests, blood gas analysis and less frequently treated patients with guideline-recommended medications. Referral to pulmonary rehabilitation was poor for both groups of clinicians. Median length of stay was shorter in GMP patients versus RS patients (3 days vs. 5 days, P = 0.001). There were no differences in the 12-month re-admission (41.7% vs. 38.5%, P = 0.664) and mortality rates (10.7% vs. 6%, P = 0.292) between both groups of patients.
CONCLUSION:
Our study found differences in the hospital AECOPD management of GMP and RS, but these did not translate into different clinical outcomes between their patients. We also found suboptimal adherence to national COPD guidelines, suggesting that there is scope for improvement in the AECOPD management of both groups of clinicians.
Keywords: Chronic obstructive pulmonary disease, disease exacerbation, guideline, hospitalization, specialization
Acute exacerbations of chronic obstructive pulmonary disease (AECOPD) is defined as an acute event characterized by a worsening of respiratory symptoms that is beyond normal day-to-day variation and leads to a change in medications.[1] Hospital admission is often required for the management of AECOPD.[1] Indeed COPD can account for 10% of all hospital admissions.[2] Across Australia in 2010-2011, there were 49,191 COPD related hospitalizations with an average length of stay of 5.1 days.[3] Hospitalization for AECOPD imposes a considerable economic burden accounting for 63% of total health expenditure for COPD patients in Australia.[3]
Among the 752 public hospitals in Australia, 34% of all hospitalizations occur in institutions which do not have full-time access to specialists.[4] In such circumstances and due to the huge overall burden of illness, general medicine physicians (GMP), rather than respiratory specialists (RS) supervise the management of hospitalized AECOPD patients. Clinical guidelines have been developed to assist clinicians in providing evidence-based management for AECOPD patients. Most prominent among them are the global initiative for COPD (GOLD), National Institute for Clinical Excellence clinical guideline on management of COPD[1,5] and the COPD-X plan (specifically for Australia and New Zealand).[6] Adherence to guideline based management of AECOPD is expected to achieve good outcomes for the immediate treatment of the exacerbation but also long-term outcomes such as survival, quality of life, reduced morbidity and reduction in decline of lung function.[7] However, adherence to guidelines in the management of hospitalized AECOPD patients has generally been suboptimal.[8] Three studies have evaluated the management of AECOPD patients between RS and GMP.[9–11] These studies revealed significant differences in the hospital management and discharge therapies between the two groups of clinicians. More recently, there is increasing interest among hospitals to develop acute medical assessment units (AMAU) since they have been shown to reduce the length of stay and emergency department waiting times for patients.[12] Typically, an AMAU is interposed between the emergency department and inpatient units and provides early review of patients by a consultant physician (usually a GMP) and an appropriately skilled multidisciplinary treatment team of nurses and allied health staff. The two campuses in our health service district are in the process of initiating AMAUs, which are expected to be fully operational by the end of 2013, and it is anticipated that the majority of AECOPD patients will be managed in the AMAU. The aims of our study were to firstly compare the management and outcome of hospitalized AECOPD patients of GMP and RS and secondly evaluate the adherence of both groups of clinicians to the COPD-X plan, the national AECOPD recommendations that were available at the time of the study period.[6]
Methods
A retrospective, observational cohort study was performed consisting of consecutive patients who had been admitted for management of AECOPD at the two campuses of the Gold Coast Health Service District (Gold Coast Hospital and Robina Hospital), Gold Coast, Queensland, Australia. The Gold Coast Health Service District provides care for 500,000 residents. Both campuses have intensive care facilities but only the Gold Coast Hospital has full-time access to RS. During the study period, there were few other differences between the two campuses. Doctors (junior doctors and general medicine specialists) and nurses were rostered interchangeably to work at both campuses. At both campuses, all medical entries were made in hard-copy medical case notes and the radiology and pathology data was available on a centralized database. Included in the study were adult patients (age ≥18 years) diagnosed with COPD by a medical practitioner, who had been hospitalized during the 2010 winter season corresponding to a 3-month period (June 2010-September 2010). COPD patients were taken to the emergency department (either Gold Coast Hospital or Robina Hospital) by ambulance staff based on an assessment of proximity to the hospital. Patients with the International Classification of Diseases-10 (ICD-10) code of E65A and E65B (complex and non-complex COPD) at the time of discharge were enrolled. Patients’ hard copy medical records pertaining to the hospital admission and electronic discharge summaries were reviewed. Patients with COPD, whose primary diagnosis for admission was not an acute exacerbation, were excluded from the study. Biochemistry results were obtained through the pathology laboratory information system. We collected information pertaining to patient demographics (age, gender, smoking status, living situation and presence of other co-existing illnesses), characteristics of underlying disease (pulmonary function tests, oxygen therapy at home and COPD-related hospital admissions in the preceding 12 months), investigations performed at the time of hospital admission (chest X-ray, full blood counts, biochemistry, blood gas analysis), treatments provided in hospital (oxygen, systemic steroids, antibiotics and bronchodilators), discharge therapy (oxygen, systemic steroids, antibiotics and bronchodilators) and outcomes (death during hospital, COPD-related hospital admission within 12-month of discharge and death from any cause within 12-month of discharge). Spirometer results were recorded if they had been performed either during the admission or during the 5-year period prior to admission. Where available, we recorded the oxygen delivery while the patient was in hospital and categorized it based on the flow rates of either ≤4L/min or >4L/min. In-hospital mortality and length of hospital stay were determined for each patient. Follow-up, survival and hospital readmission for AECOPD data was collected for up to 12-month following initial hospital discharge of all patients by review of hardcopy and electronic medical records. Adherence to the COPD-X plan regarding the evaluation and treatment of acute exacerbations of COPD was assessed.[13] The study was approved by the Gold Coast Health Service District Human Research Ethics Committee (HREC/12/QGC/57).
Statistical analysis
All statistical analyses were performed using Statistical Product and Service Solutions (SPSS) Statistics version 20.0 (SPSS, Chicago, IL, USA). The variables included in the study were used to compare the group of patients that were managed by GMP compared to those managed by RS. For data that were normally distributed, analyses of differences between individual groups were assessed by the Student's t-test and presented as mean and standard error. Mann-Whitney U test was used to determine if there were differences between groups and results presented as median and range. Logistic regression analysis was used to understand the relationship between the studied variables and the two groups of patients. Variables from the univariate analysis that had a P < 0.2 were evaluated in the multivariate regression analysis. All results with a P < 0.05 were deemed to be statistically significant.
Results
During the 3-month study period, 257 presentations were identified using the ICD-10 codes. After review of the medical case records, 56 presentations were excluded from analysis as they were found to be either incorrectly coded or AECOPD was not the primary reason for hospitalization. Final analysis was performed on 201 AECOPD presentations in 169 patients. Over the 3-month, 25 (12.4%) patients were admitted twice, 6 (3%) patients were admitted 3 times and 1 (0.5%) patient was admitted 4 times. Patient characteristics are presented in Table 1. The mean age of the patients was 72.3 years and females accounted for 50.3% of the study cohort. 65% of patients were either current or former smokers. Among the common co-morbid illnesses documented, ischemic heart disease was the most common (33%). Twenty percent of the patients were using home oxygen at the time of hospital admission. Thirty one percent of patients had been admitted to hospital for management of AECOPD at least once in the previous 12-month.
Table 1.
Patient characteristics
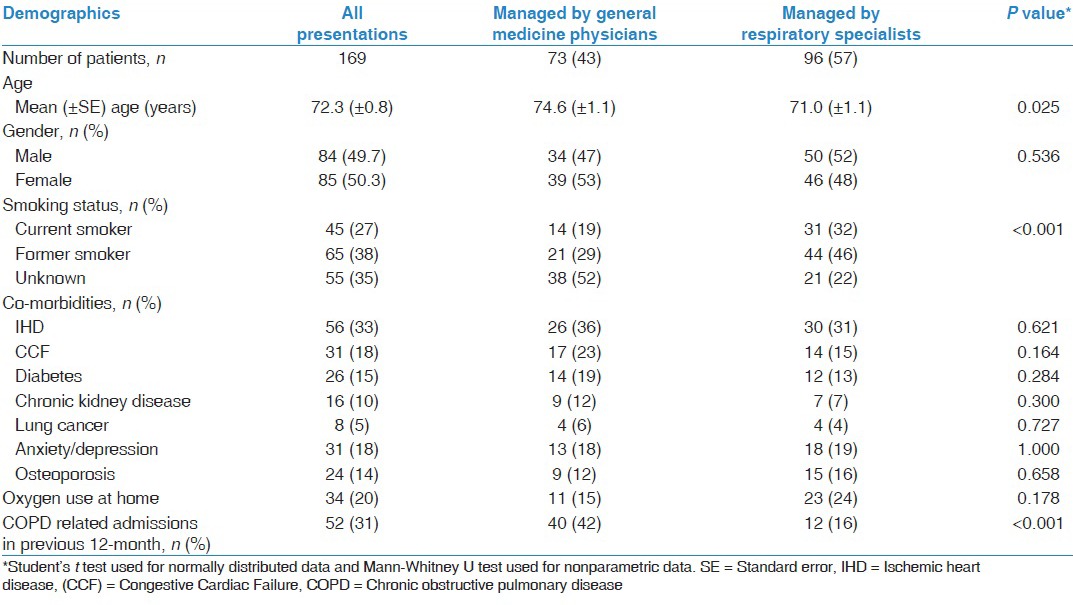
During the study period, 42% of AECOPD presentations were managed by GMP [Table 2]. The sex distribution, co-morbid illness and home oxygen use was similar between GMP patients and RS patients. GMP patients were older than RS patients (74.6 years vs. 71.0 years, P = 0.025). We found that smoking status was not documented more commonly in the GMP patients compared to RS patients (52% vs. 22%, P < 0.001). Spirometry results were less frequently available in the notes of GMP patients compared to RS patients (10% vs. 39%, P < 0.001). An assessment of acid base status using either an arterial or venous blood gas analysis was performed less frequently in GMP patients compared to RS patients (39.3% vs. 77.8%, P < 0.001).
Table 2.
Investigations and treatment during hospital admission
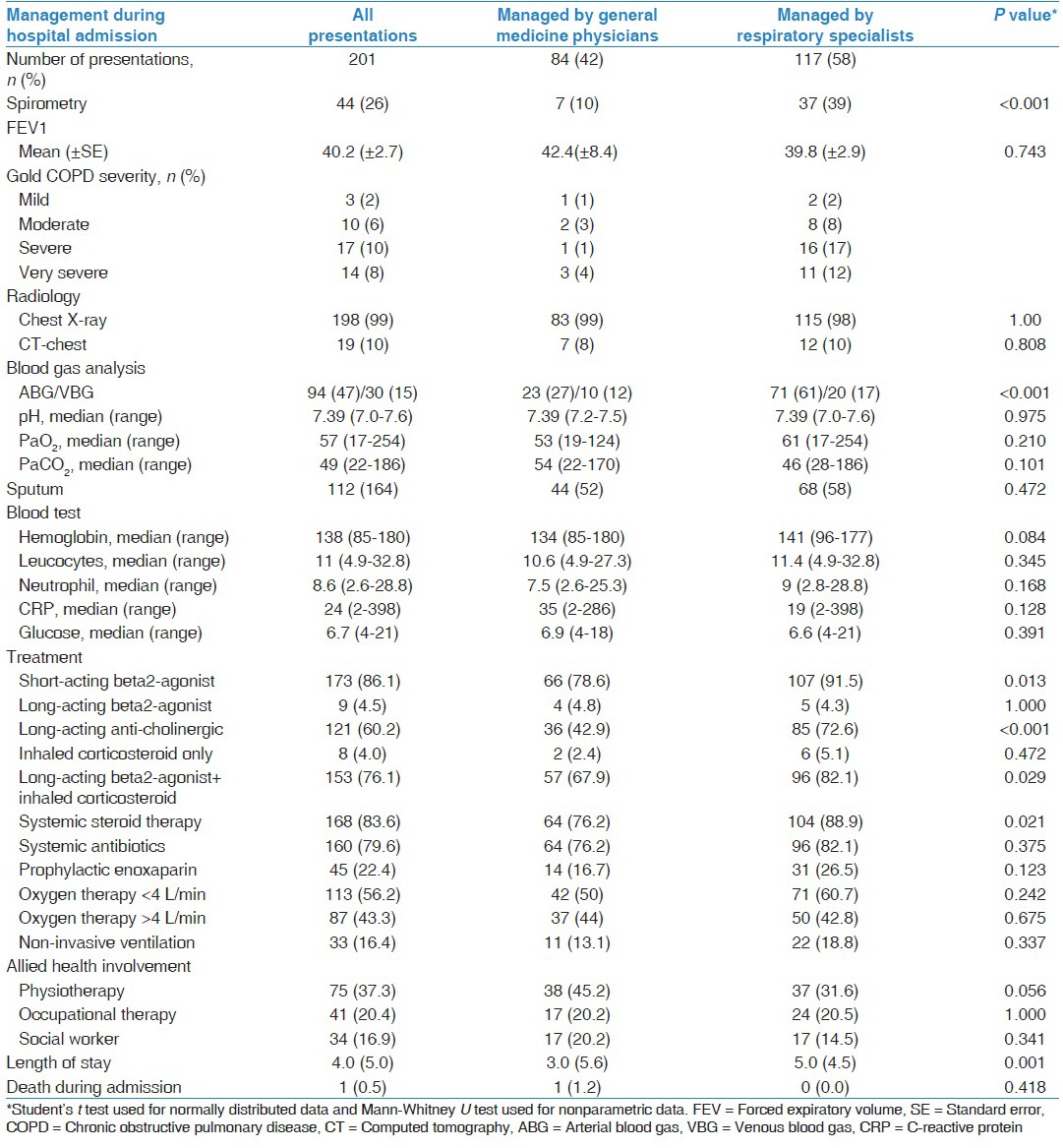
Twelve GMP patients had hypercapneic respiratory failure (pH < 7.35 and PaCO2 > 45 mmHg), but 2 (17%) received non-invasive ventilation (NIV) while 24 RS patients had hypercapneic respiratory failure and NIV was provided to 9 (39%) patients. There was no documentation about the reasons why NIV was not provided to the other eligible patients. During hospitalization, GMP patients less frequently received short-acting beta2-agonist (78.6% vs. 91.5%, P = 0.013), long-acting anti-cholinergic (42.9% vs. 72.6%, P < 0.001), combination of long-acting beta2-agonist and inhaled corticosteroid therapy (67.9% vs. 82.1%, P = 0.029) and systemic steroid therapy (76.2% vs. 88.9%, P = 0.021). Multivariate analysis demonstrated that during hospitalization, the only difference in management by RS in comparison to GMP, is that RS performed spirometry (odds ratio [OR] 4.7, 95% confidence interval [CI] [1.88-11.82], P < 0.001) and blood gas analysis tests (OR 2.2, 95% CI [1.49-3.23], P < 0.001) more frequently. The median length of stay was shorter in the GMP patients compared to RS patients (3 days vs. 5 days, P < 0.001).
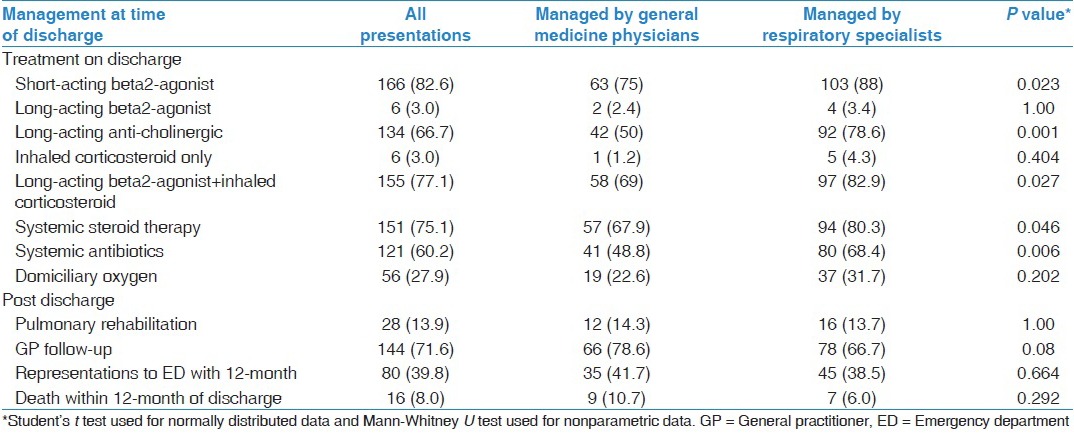
There were similar differences in the medications that patients were prescribed at the time of hospital discharge between the two groups of patients [Table 3]. GMP patients were discharged with the following medications less frequently than RS patients: Short-acting beta2-agonist (75% vs. 88%, P = 0.023), long-acting anti-cholinergic (50% vs. 78.6%, P = 0.001), combination of long-acting beta2-agonist and inhaled corticosteroid therapy (69% vs. 82.9%, P = 0.027), systemic steroid therapy (67.9% vs. 80.3%, P = 0.046) and systemic antibiotics (48.8% vs. 68.4%, P = 0.006). Fifty-six patients (27.9%) were discharged with supplemental oxygen of which 14 patients were not previously on home oxygen therapy. Among the 14, an arterial blood gas (ABG) result was available in 8 patients (57%). The referral rate to pulmonary rehabilitation was low in both groups of patients (RS-13.7% and GMP-14.3%). Multivariate analysis demonstrated that at the time of discharge, RS in comparison to GMP, prescribed long-acting anti-cholinergics (OR 2.31, 95% CI [1.01-4.23], P = 0.049) and antibiotics (OR 2.2, 95% CI [1.13-3.23], P = 0.021) more frequently and were less likely to arrange General Practitioner (GP) follow-up for their patients (OR 0.34, 95% CI [0.15-0.74], P = 0.006). There was no differences in the AECOPD related hospitalization (41.7% vs. 38.5%, P = 0.664) or overall mortality (10.7% vs. 6%, P = 0.292) within 12-months of hospital discharge between the GMP and RS patients.
Table 3.
Discharge therapy and outcomes
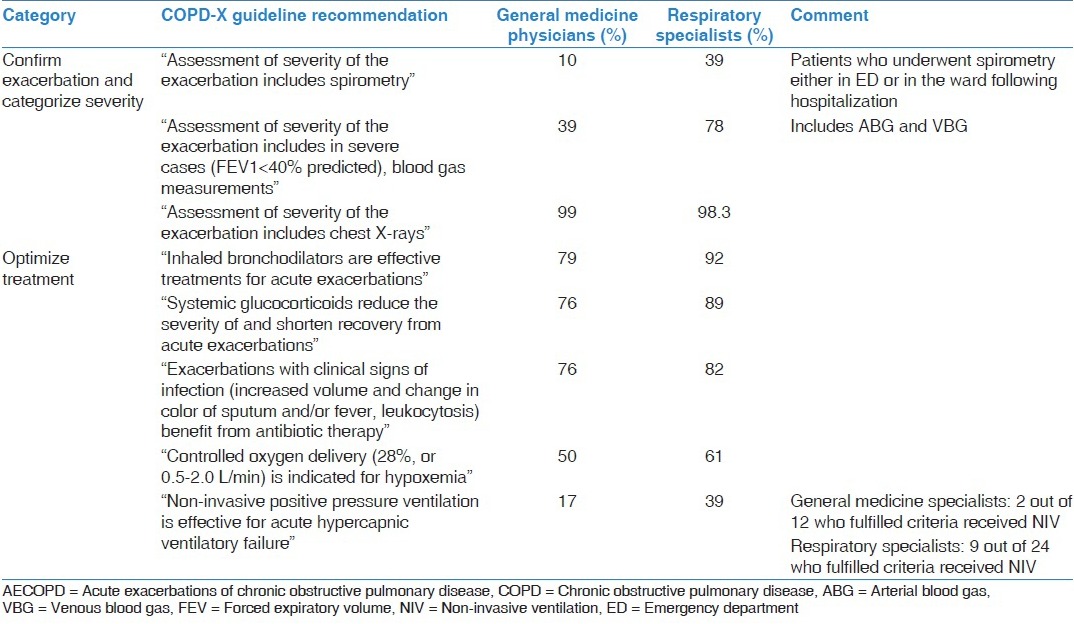
Overall, adherence to the COPD-X plan guideline was good [Table 4]. Adherence to guideline recommendations regarding chest radiographs was excellent. With regards to guideline recommended treatment, the vast majority of patients received bronchodilators, systemic corticosteroids and antibiotics. However, objective measurement of blood gas was performed in a small proportion of GMP patients compared to RS patients. Additionally, pulmonary function was available in only a small proportion of patients. Similarly, NIV was provided in only a small proportion of eligible patients by both groups of clinicians.
Table 4.
Observed frequencies for diagnostic procedures and clinical management of AECOPD patients
Discussion
The purpose of the present study was to explore differences in the management of AECOPD patients between two groups of specialists (GMP and RS) and to audit their practices against national COPD management guidelines. We found that GMP performed fewer investigations and provided less pharmacological management (while in hospital and at discharge) compared with RS. The length of stay in hospital was shorter in GMP patients and importantly there were no differences in the 12-month outcomes studied. We also noted that there was suboptimal adherence to COPD management guidelines by both GMP and RS, particularly in relation to the provision of NIV to eligible patients and referral to pulmonary rehabilitation.
In our study, we found that with regards to pharmacological management in hospital, there were notable differences in the management between GMP and RS. GMP did not prescribe short-acting beta-agonists, long-acting anti-cholinergics, combination LABA/ICS and systemic steroids as frequently as RS and instead prescribed short-acting anti-cholinergics more frequently. Notably, previous studies have also identified that systemic steroids are not as frequently prescribed by GMP compared with RS.[14] The practice of RS prescribing more medications at the time of discharge has been noted previously.[10] Providing a longer duration of corticosteroids and antibiotics is concordant with AECOPD management guidelines, which recommend that oral steroids and antibiotics should be used for 10-14 days.[1,6] However, a recent study demonstrated that in patients with AECOPD, a 5 days treatment with systemic glucocorticoids was non-inferior to 14 days treatment with regard to re-exacerbation within 6 months.[15] These results may explain why we did not find a difference in 12-month readmission rate between GMP and RS.
Both international and national COPD management guidelines, emphasize the importance of performing spirometry in establishing the diagnosis of COPD and determining the severity of illness.[1,6] In our study, spirometry results were available in 26% of all patients, with the tests being performed more frequently by RS compared to GMP (39% vs. 10%, P < 0.001). This finding is consistent with previous studies, which have also demonstrated higher frequency of spiromtery testing by RS.[8,16,17] While previous audit studies of COPD patients have found suboptimal performance of spirometry,[10,11,16–18] Chang et al. had spirometry rates performed in 94% of their study cohort.[9] This demonstrates that high rate of concordance with COPD guidelines can be achieved if there is adequate awareness among clinicians and local policies to implement the COPD guideline recommendations.
Another key recommendation of the COPD-X plan is that ABG must be performed in patients with a percutaneous oxygen saturation of <92% or a forced expiratory volume in 1 s of less than 1 L or 40% predicted.[6] An assessment of acidosis and hypercapnia using either a venous blood gas or ABG analysis performed in only 39% of GMP patients compared to RS patients, where it was performed in 78% of patients. It is important to evaluate the acid-base status since in patients with hypercapneic respiratory failure, the provision of NIV has been demonstrated to improve survival, reduce the length of stay, mortality and need for intubation.[19] However, NIV therapy was provided to only 33% of patients who fulfilled criteria and there was no difference between RS and GMS in the provision of NIV. This is similar to other studies, which have reported that only one-third of acidotic COPD patients receive NIV, often with no documentation as to why NIV was not offered to other eligible patients.[17,20] Further study is required to better understand the reasons for poor prescription of NIV for eligible patients.
Randomized controlled studies have found that pulmonary rehabilitation is highly effective and safe intervention to reduce hospital admissions and mortality and to improve the health-related quality of life in COPD.[21] We found no difference in referral for pulmonary rehabilitation between GMP and RS patients, which in comparison to guidelines was suboptimal. Indeed, previous audits have also found that referral rates for pulmonary rehabilitation are similar to our study.[8] Since our study was a retrospective audit, we were unable to determine the reasons for the low referral rate. Studies which have evaluated the reasons for non-referral to rehabilitation by interviewing potential referring practitioners have reported that the barriers to referral are often complex and include factors such as low awareness, low support for rehabilitation at multiple levels, lack of time and a perceived difficult referral process.[22,23]
In our study, GMP patients were admitted for a shorter period than RS patients. Interestingly two previous studies did not identify any difference in length of stay LOS between RS and GMP patients.[11,16] Price et al. compared LOS across several UK hospitals and found wide variations in LOS across the hospitals, which was attributed to the staffing and resources rather than the management patterns of individual physicians.[2] We evaluated the hospital re-admission and mortality rates at the 12-month post the index AECOPD admission and found no differences between the GMP and RS managed patient cohorts. Similar to our study, Angus et al. found that when 12-month readmission or mortality rates are evaluated, there were no differences between RS and GMP patients.[16]
Our study is prone to the limitations of retrospective review of medical records, with the assumption that documentation is an accurate reflection of clinical practice.[24] We studied the admissions to our institution over a 3-month period covering the winter months, which may not reflect the practice and resources during other periods of the year. Furthermore, while the findings of our study are generally similar to other previous audits of COPD patients, it should be noted that the results of our study may not necessarily reflect the practice patterns outside of our institutions. Hence, the results of our study may not be generalizable to other health care institutions. In order to study the management of AECOPD patients, a prospective multi-center audit is required, similar to the recently proposed European COPD Audit.[25]
Conclusion
By undertaking this study, we now have a better understanding of the patterns of care that has been provided to AECOPD patients in our health service district. We found differences in the management practices of hospitalized AECOPD patients between RS and GMP but these differences did not translate into outcome differences for their respective patients. In general, management of RS was more in accordance with national COPD guidelines, but GMP patients had shorter lengths of stay in hospital. The results of our study demonstrate that there is an opportunity for cross-fertilization of knowledge and practice patterns across clinicians so that all patients receive evidence-based care in the most efficient manner.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–55. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 2.Price LC, Lowe D, Hosker HS, Anstey K, Pearson MG, Roberts CM, et al. UK National COPD Audit 2003: Impact of hospital resources and organisation of care on patient outcome following admission for acute COPD exacerbation. Thorax. 2006;61:837–42. doi: 10.1136/thx.2005.049940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Access Economics. Economic Impact of COPD and Cost Effective Solutions. ACT: The Australian Lung Foundation. 2008 [Google Scholar]
- 4.43rd ed. Canberra: AIHW; 2012. AIHW. Australian Hospital Statistics 2010-11. [Google Scholar]
- 5.National Institute for Clinical Excellence. CG101 chronic obstructive pulmonary disease–full guideline. [Last accessed on 2013 Mar 1]. Available from: http://www.nice.org.uk/page.aspx?o_107650 .
- 6.McKenzie DK, Frith PA, Burdon JG, Town GI. Australian Lung Foundation, Thoracic Society of Australia and New Zealand. The COPDX Plan: Australian and New Zealand guidelines for the management of chronic obstructive pulmonary disease 2003. Med J Aust. 2003;178:S7–39. doi: 10.5694/j.1326-5377.2003.tb05213.x. [DOI] [PubMed] [Google Scholar]
- 7.Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet. 2012;379:1341–51. doi: 10.1016/S0140-6736(11)60968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lodewijckx C, Sermeus W, Vanhaecht K, Panella M, Deneckere S, Leigheb F, et al. Inhospital management of COPD exacerbations: A systematic review of the literature with regard to adherence to international guidelines. J Eval Clin Pract. 2009;15:1101–10. doi: 10.1111/j.1365-2753.2009.01305.x. [DOI] [PubMed] [Google Scholar]
- 9.Chang CL, Sullivan GD, Karalus NC, Hancox RJ, McLachlan JD, Mills GD. Audit of acute admissions of chronic obstructive pulmonary disease: Inpatient management and outcome. Intern Med J. 2007;37:236–41. doi: 10.1111/j.1445-5994.2006.01283.x. [DOI] [PubMed] [Google Scholar]
- 10.Roberts CM, Ryland I, Lowe D, Kelly Y, Bucknall CE, Pearson MG, et al. Audit of acute admissions of COPD: Standards of care and management in the hospital setting. Eur Respir J. 2001;17:343–9. doi: 10.1183/09031936.01.17303430. [DOI] [PubMed] [Google Scholar]
- 11.Kelly MG, Elborn JS. British Thoracic Society. Admissions with chronic obstructive pulmonary disease after publication of national guidelines. Ir J Med Sci. 2002;171:16–9. doi: 10.1007/BF03168933. [DOI] [PubMed] [Google Scholar]
- 12.Li JY, Yong TY, Bennett DM, O’Brien LT, Roberts S, Hakendorf P, et al. Outcomes of establishing an acute assessment unit in the general medical service of a tertiary teaching hospital. Med J Aust. 2010;192:384–7. doi: 10.5694/j.1326-5377.2010.tb03560.x. [DOI] [PubMed] [Google Scholar]
- 13.David McKenzie K, Michael Abramson, Alan Crockett J, Nicholas Glasgow, Sue Jenkins, Christine McDonald, Richard Wood-Baker. Peter A Frith on behalf of The Australian Lung Foundation. The COPD-X Plan: Australian and New Zealand Guidelines for the management of Chronic Obstructive Pulmonary Disease. 2010 [Google Scholar]
- 14.Neill AM, Epton MJ, Martin IR, Drennan CJ, Town GI. An audit of the assessment and management of patients admitted to Christchurch Hospital with chronic obstructive pulmonary disease. N Z Med J. 1994;107:365–7. [PubMed] [Google Scholar]
- 15.Leuppi JD, Schuetz P, Bingisser R, Bodmer M, Briel M, Drescher T, et al. Short-term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: The REDUCE randomized clinical trial. JAMA. 2013;309:2223–31. doi: 10.1001/jama.2013.5023. [DOI] [PubMed] [Google Scholar]
- 16.Angus RM, Murray S, Kay JW, Thomson NC, Patel KR. Management of chronic airflow obstruction: Differences in practice between respiratory and general physicians. Respir Med. 1994;88:493–7. doi: 10.1016/s0954-6111(05)80329-4. [DOI] [PubMed] [Google Scholar]
- 17.Pretto JJ, McDonald VM, Wark PA, Hensley MJ. Multicentre audit of inpatient management of acute exacerbations of chronic obstructive pulmonary disease: Comparison with clinical guidelines. Intern Med J. 2012;42:380–7. doi: 10.1111/j.1445-5994.2011.02475.x. [DOI] [PubMed] [Google Scholar]
- 18.Ta M, George J. Management of chronic obstructive pulmonary disease in Australia after the publication of national guidelines. Intern Med J. 2011;41:263–70. doi: 10.1111/j.1445-5994.2009.02133.x. [DOI] [PubMed] [Google Scholar]
- 19.Ram FS, Picot J, Lightowler J, Wedzicha JA. Non-invasive positive pressure ventilation for treatment of respiratory failure due to exacerbations of chronic obstructive pulmonary disease [review] Cochrane Database Syst Rev. 2004;3 doi: 10.1002/14651858.CD004104.pub3. CD004104. [DOI] [PubMed] [Google Scholar]
- 20.Hosker H, Anstey K, Lowe D, Pearson M, Roberts CM. Variability in the organisation and management of hospital care for COPD exacerbations in the UK. Respir Med. 2007;101:754–61. doi: 10.1016/j.rmed.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 21.Puhan MA, Gimeno-Santos E, Scharplatz M, Troosters T, Walters EH, Steurer J. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011;5 doi: 10.1002/14651858.CD005305.pub3. CD005305. [DOI] [PubMed] [Google Scholar]
- 22.Johnston K, Grimmer-Somers K, Young M, Antic R, Frith P. Which chronic obstructive pulmonary disease care recommendations have low implementation and why? A pilot study. BMC Res Notes. 2012;5:652. doi: 10.1186/1756-0500-5-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris D, Hayter M, Allender S. Factors affecting the offer of pulmonary rehabilitation to patients with chronic obstructive pulmonary disease by primary care professionals: A qualitative study. Prim Health Care Res Dev. 2008;9:280–90. [Google Scholar]
- 24.Hirschtick RE. A piece of my mind. John Lennon’s elbow. JAMA. 2012;308:463–4. doi: 10.1001/jama.2012.8331. [DOI] [PubMed] [Google Scholar]
- 25.López-Campos JL, Hartl S, Pozo-Rodriguez F, Roberts CM. European COPD Audit team. European COPD Audit: Design, organisation of work and methodology. Eur Respir J. 2013;41:270–6. doi: 10.1183/09031936.00021812. [DOI] [PubMed] [Google Scholar]


