Abstract
BACKGROUND:
Exposure to environmental tobacco smoke (ETS) has harmful effects on the pregnancy outcomes similar to those observed in actively smoking pregnant women. The aim of this study was to estimate the sensitivity and specificity of the breath carbon monoxide (BCO) analysis in the assessment of smoking status among Saudi pregnant women, including ETS exposure compared to self-reported tobacco smoke exposure.
METHODS:
A cross-sectional design was used during January 2012, 560 pregnant women, irrespective of their gestational age, agreed to undergo BCO testing and completed the data collection sheet for the study. Sensitivity, specificity, positive and negative predictive values were calculated to compare the BCO test with self-reported exposure to ETS.
RESULTS:
Of the study population 151 (27%) women self-reported ETS exposure during the index pregnancy, 409 (73%) self-reported non-exposure. Sensitivity of the test was 32.5% (95% CI; 25.2-40.3%), the Specificity was much higher at 69.2% (95% CI; 64.4-73.5%), the positive predictive value was 28% (95% CI, 21.9-35.1%), and the negative predictive value was 73.5% (95% CI; 68.9-77.7%).
CONCLUSION:
The BCO test is an ineffective tool to detect the level of ETS exposure among Saudi pregnant women.
Keywords: Breath carbon monoxide analyzer, environmental tobacco smoke, pregnancy, Saudi Arabia
Environmental tobacco smoke (ETS) exposure is one of the major public health problems given that 22-30% of non-smoking pregnant women were reported to be exposed to ETS.[1] A study from the Eastern Province of Saudi Arabia reported that 50% of pregnant women had been exposed to ETS.[2] Exposure to ETS of non-smoking pregnant women has harmful effects on pregnancy outcomes comparable to those observed in actively smoking pregnant women, including; stillbirth, congenital malformations, low birth weight, intrauterine growth restriction, sudden infant death syndrome, miscarriage, and preterm delivery.[3–9]
Various methods were cited in the literature to assess tobacco exposure, such as assays of cotinine (the major metabolite of nicotine) in urine and blood, hair nicotine, breath carbon monoxide analysis (BCO) and self-reporting of tobacco exposure. Cotinine test is considered the most popular and accurate measure of the tobacco smoking and ETS exposure as it has a longer half-life of 17 h in non-pregnant populations[10] and 9 h in pregnant women.[11] Hair analysis for nicotine is another non-invasive test of the long-term accumulation of tobacco exposure.[12] On the other hand, BCO analyzer is an immediate, non-invasive and affordable tool used to assess active smoking and ETS status and is appropriate for assessing smoking status in a wide variety of clinical settings including antenatal clinics.[13,14] BCO analyzer has different cut-off points in different populations, based on the intended use of the tool, which can be used for assessing: Antenatal smoking;[15] secondhand smoke;[16] in the clinical and community-based surveys[17] as well as validating smoking cessation.[18]
Self-reported smoking has been used in many studies to evaluate the smoking status in different population.[19,20] However, this method is likely to under-estimate the smoking exposure in certain communities due to societal unacceptability or among a certain group of people including pregnant women and parents of young children.[21]
Pregnancy is known to affect the maternal metabolism, which leads to accelerated clearance of many substances, including nicotine, compared to non-pregnant women.[22] Hence, the short half-life of cotinine in pregnant women and the shorter window for detecting the substance in the breath after the last exposure.[23]
This study aimed at estimating the sensitivity and specificity of using the BCO analysis in the assessment of smoking status among Saudi pregnant women, including ETS exposure compared to self-reported tobacco smoke exposure.
Methods
This study was conducted at the antenatal clinics of a university hospital, Riyadh, Saudi Arabia. It is a tertiary referral hospital with 750 bed-capacity. The obstetrics department includes a neonatal intensive care unit, in-vitro fertilization and maternal and fetal medicine units.
Using a cross-sectional design during January 2012, 560 pregnant women, irrespective of their gestational age, agreed to undergo BCO testing and completed the data collection sheet for the study. Two staff nurses from the obstetric outpatient department were trained by the researchers; on the objectives of this study and how to conduct BCO testing. Consequently, they assisted participants incompleting the data collection sheet and BCO testing, which was measured using (BMC-2000) analyzer.[24]
The data collection sheet was designed in three parts. The first part included the demographic data (age, level of education; occupational status…, etc.). The second part collected data about exposure to ETS, which was defined as occurring when a woman, who did not smoke at all whilst pregnant, lived with a household member (husband, son, daughter or other relatives) who reported smoking during the index pregnancy. The third part collected information about the status of tobacco exposure, women who self-reported smoking were excluded from the study. Occupational exposure was not assessed as only small percentage of the cohort was working for pay and the Kingdom legislation bans smoking in the work place. In addition, the duration of exposure to ETS was not reported since only 20% of the women could recall the duration of exposure.
Ten minutes after completing the questionnaire and explaining BCO testing procedures, women were asked to perform the test. They were asked to exhale completely, inhale fully, hold their breath for 15 second and then exhale slowly into the BMC-2000 chamber. The BMC reading was then registered and reported as parts per million (PPM).
Data analysis
The BCO test was considered negative if the PPM was zero, whilst any reading above zero was considered as positive. Sensitivity was defined as the proportion of all self-reported ETS exposed for whom there was a positive BCO test result while specificity was defined as the proportion of all self-reported non ETS exposed for whom there was a negative BCO test result. Positive predictive value (PPV) is the probability that women with a positive PPM test truly exposed to ETS. Negative predictive value (NPV) is the probability that women with a negative test were truly unexposed to ETS. Sensitivity, specificity, positive and negative predicted values were calculated through knowledge translation clearinghouse website, center for Evidence Based Medicine Toronto.[25] Statistical Package for Social Sciences (SPSS), namely PASW statistics data document 18, was used for other descriptive statistics.
All ethical considerations were observed, including protection of confidentiality of information and anonymity of participants. The approval of the Institutional Review Board of College of medicine, King Saud University was obtained (numbered E-11-363), before commencing the study.
Results
During the study period, 1,636 pregnant women were seen at the antenatal clinic and 560 women consented to the study. All participants had both self-reported tobacco exposure and BCO testing data. The mean age of the cohort was 28 ± 6.19 years. Only 15 women were illiterate while the rest had formal education and more than half had university and above education (51.6%). Of the participants, 73% were housewives [Table 1].
Table 1.
Demographic characteristics of pregnant women and the exposure to environmental tobacco smoke (n=560)
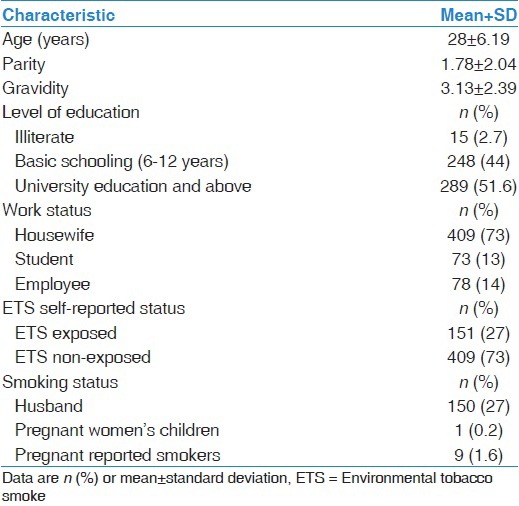
Of the study population, 151 (27%) women self-reported ETS exposure during the index pregnancy, 409 (73%) self-reported non-exposure and 9 (1.6%) women were current active smokers and were excluded from further analysis [Table 1].
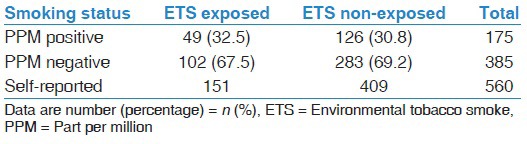
Table 2 showed that out of 151 women who reported ETS exposure, only 49 (32.5%) had positive PPM test results while 126 women had false positive results. On the other hand, 283 women out of 409 (69%) who reported non-exposure to ETS, had negative PPM results while 102 women had false negative results.
Table 2.
Readings of breath carbon monoxide testing of pregnant women who were exposed or not exposed to environmental tobacco smoke (n=560)
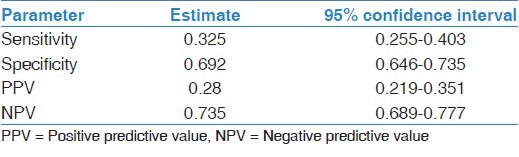
Sensitivity of the test was 32.5% (95% confidence interval [CI]; 25.2-40.3%), which means that BCO analysis could correctly detect about 33% of self-reported ETS exposed pregnant women. The specificity was much higher at 69.2% (95% CI; 64.4-73.5%) meaning that the probability of BCO test correctly identifying subjects not exposed to ETS was 69.2%. Among those who had a positive BCO test, the probability of ETS exposure was 28% (PPV = 28%, 95% CI, 21.9-35.1%) while among those who had a negative BCO test, the probability of being not exposed to ETS was 73.5% (NPV = 73.5%, 95% CI; 68.9-77.7%) [Table 3].
Table 3.
Sensitivity and specificity of breath carbon monoxide and 95% confidence interval
Discussion
The result of this study showed that BCO analysis had a sensitivity of 33%, which means that the test was able to detect only one third of the population who reported exposure to the ETS; however, the test had a better specificity of 69.2% meaning that the probability of the test to identify ETS non exposure was 69%. The modest performance of the BCO analyzer in detecting ETS exposure may be attributed to many factors related to the physiological changes of pregnancy, the metabolism of nicotine in the human breath and the ability of the BCO analyzer to detect the relatively low levels of ETS compared to those of active smokers.
The physiological changes of pregnancy alter the clearance of some drugs and chemicals from the body including cotinine,[22,26] which results in a short half-life of Carbon Monoxide (CO), secondary to exposure to ETS, in the exhaled air of the pregnant woman and subsequently a narrow window for detection. The attendance of the pregnant women to the antenatal clinic, especially for the morning clinic, after an overnight sleep made the interval between last exposure and the BCO test long, which explains the high rate of false negative results.[13]
Another reason for the poor performance of CO analysis in screening for ETS exposure, is the inability of the BCO analyzer to detect low levels of tobacco exposure;[14] it has been confirmed that exposure to ETS results in far lower levels of nicotine in the blood and subsequent CO in the breath compared with active tobacco smoking,[27] which may explain the low positive and negative predicative values for this test.
The findings of this study confirmed that self-reported exposure is more reliable than the BCO testing in the detection of the Saudi pregnant women who were exposed to ETS and hence can be used for screening to facilitate health education to reduce the harm of exposure. However, for more evaluation of impact of measure to reduce ETS exposure among pregnant Saudi women, more robust biomarker detection test to quantifying nicotine exposure in the hair, blood, urine or saliva are needed.[10,12]
One-third of the study population were exposed to domestic ETS, this result was similar to other studies[28–30] but less than other cohort with an estimated exposure of 52%.[31] Previous studies have demonstrated the pivotal role of knowledge about the harmful effects of tobacco use on pregnancy outcomes as a base for positive attitude toward the practice of avoidance ETS exposure.[32–34] Antenatal setting represents a great opportunity for obstetricians and other health-care providers to campaign for and raise awareness of pregnant women to smoking cessation and avoidance of ETS exposure, because women frequently visit the clinic during their pregnancy and can be monitored easily.[35,36] Studies showed that 15-40% of women who smoked when not pregnant, spontaneously quit smoking during pregnancy.[37] Moreover, a study from Iran confirmed that the health education on the harmful effects of ETS exposure was associated with a reduction of ETS exposure among pregnant women.[38]
It is worth noting that the majority of this study population (96%) had formal education, which makes the utilization of written educational material and internet based health education a viable option.
We are aware of the limitations of this study including the use of self-reported ETS rather than biomarkers as a gold standard; however, we believe that self-reported exposure to ETS may be considered reliable in this cohort, as it is not associated with social unacceptability, similar to active tobacco smoking. The occupational ETS exposure was not assessed in this study. However, this might not have affected the results because of the low percentage of respondents who reported working for pay, in addition to the fact that, there is gender segregation in most of Saudi workplaces and the recent implementation of the legislation which bans smoking in the public places.[39,40]
Conclusion
The BCO test is an ineffective tool to detect the level of ETS exposure among Saudi pregnant women in this study.
Acknowledgments
We would like to acknowledge the assistance of Mrs. Kamelia Dawood and Rosalia Mahmud of the antenatal ward for their participation in the data collection and BCO tests for this study.
Footnotes
Source of Support: This study was funded by grand (#RGP-VPP-149 date 4/6/1432) from the Deanship of Scientific Research, King Saud University, Riyadh, KSA.
Conflict of Interest: None declared.
References
- 1.Shah CP. Public Health and Preventative Medicine in Canada. 5th ed. Toronto, Canada: Elsevier; 2003. p. 201. [Google Scholar]
- 2.Rashid M, Rashid H. Passive maternal smoking and pregnancy outcome in a Saudi population. Saudi Med J. 2003;24:248–53. [PubMed] [Google Scholar]
- 3.Leonardi-Bee J, Britton J, Venn A. Secondhand smoke and adverse fetal outcomes in nonsmoking pregnant women: A meta-analysis. Pediatrics. 2011;127:734–41. doi: 10.1542/peds.2010-3041. [DOI] [PubMed] [Google Scholar]
- 4.Leonardi-Bee J, Smyth A, Britton J, Coleman T. Environmental tobacco smoke and fetal health: Systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2008;93:F351–61. doi: 10.1136/adc.2007.133553. [DOI] [PubMed] [Google Scholar]
- 5.Salmasi G, Grady R, Jones J, McDonald SD Knowledge Synthesis Group. Environmental tobacco smoke exposure and perinatal outcomes: A systematic review and meta-analyses. Acta Obstet Gynecol Scand. 2010;89:423–41. doi: 10.3109/00016340903505748. [DOI] [PubMed] [Google Scholar]
- 6.Reichert VC, Seltzer V, Efferen LS, Kohn N. Women and tobacco dependence. Obstet Gynecol Clin North Am. 2009;36:877–90. doi: 10.1016/j.ogc.2009.10.003. xi. [DOI] [PubMed] [Google Scholar]
- 7.Rogers JM. Tobacco and pregnancy. Reprod Toxicol. 2009;28:152–60. doi: 10.1016/j.reprotox.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Haustein KO. Cigarette smoking, nicotine and pregnancy. Int J Clin Pharmacol Ther. 1999;37:417–27. [PubMed] [Google Scholar]
- 9.Lambers DS, Clark KE. The maternal and fetal physiologic effects of nicotine. Semin Perinatol. 1996;20:115–26. doi: 10.1016/s0146-0005(96)80079-6. [DOI] [PubMed] [Google Scholar]
- 10.Florescu A, Ferrence R, Einarson T, Selby P, Soldin O, Koren G. Methods for quantification of exposure to cigarette smoking and environmental tobacco smoke: Focus on developmental toxicology. Ther Drug Monit. 2009;31:14–30. doi: 10.1097/FTD.0b013e3181957a3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benowitz NL, Jacob P., 3rd Metabolism of nicotine to cotinine studied by a dual stable isotope method. Clin Pharmacol Ther. 1994;56:483–93. doi: 10.1038/clpt.1994.169. [DOI] [PubMed] [Google Scholar]
- 12.Eliopoulos C, Klein J, Phan MK, Knie B, Greenwald M, Chitayat D, et al. Hair concentrations of nicotine and cotinine in women and their newborn infants. JAMA. 1994;271:621–3. [PubMed] [Google Scholar]
- 13.Frederiksen LW, Martin JE. Carbon monoxide and smoking behavior. Addict Behav. 1979;4:21–30. doi: 10.1016/0306-4603(79)90017-0. [DOI] [PubMed] [Google Scholar]
- 14.Secker-Walker RH, Vacek PM, Flynn BS, Mead PB. Exhaled carbon monoxide and urinary cotinine as measures of smoking in pregnancy. Addict Behav. 1997;22:671–84. doi: 10.1016/s0306-4603(97)00013-0. [DOI] [PubMed] [Google Scholar]
- 15.Campbell E, Sanson-Fisher R, Walsh R. Smoking status in pregnant women assessment of self-report against carbon monoxide (CO) Addict Behav. 2001;26:1–9. doi: 10.1016/s0306-4603(00)00070-8. [DOI] [PubMed] [Google Scholar]
- 16.Deveci SE, Deveci F, Açik Y, Ozan AT. The measurement of exhaled carbon monoxide in healthy smokers and non-smokers. Respir Med. 2004;98:551–6. doi: 10.1016/j.rmed.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 17.Jagoe K, Edwards R, Mugusi F, Whiting D, Unwin N. Tobacco smoking in Tanzania, East Africa: Population based smoking prevalence using expired alveolar carbon monoxide as a validation tool. Tob Control. 2002;11:210–4. doi: 10.1136/tc.11.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hung J, Lin CH, Wang JD, Chan CC. Exhaled carbon monoxide level as an indicator of cigarette consumption in a workplace cessation program in Taiwan. J Formos Med Assoc. 2006;105:210–3. doi: 10.1016/S0929-6646(09)60307-7. [DOI] [PubMed] [Google Scholar]
- 19.Patrick DL, Cheadle A, Thompson DC, Diehr P, Koepsell T, Kinne S. The validity of self-reported smoking: A review and meta-analysis. Am J Public Health. 1994;84:1086–93. doi: 10.2105/ajph.84.7.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Connor Gorber S, Schofield-Hurwitz S, Hardt J, Levasseur G, Tremblay M. The accuracy of self-reported smoking: A systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob Res. 2009;11:12–24. doi: 10.1093/ntr/ntn010. [DOI] [PubMed] [Google Scholar]
- 21.Fendrich M, Mackesy-Amiti ME, Johnson TP, Hubbell A, Wislar JS. Tobacco-reporting validity in an epidemiological drug-use survey. Addict Behav. 2005;30:175–81. doi: 10.1016/j.addbeh.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Loebstein R, Lalkin A, Koren G. Pharmacokinetic changes during pregnancy and their clinical relevance. Clin Pharmacokinet. 1997;33:328–43. doi: 10.2165/00003088-199733050-00002. [DOI] [PubMed] [Google Scholar]
- 23.Dempsey D, Jacob P, 3rd, Benowitz NL. Accelerated metabolism of nicotine and cotinine in pregnant smokers. J Pharmacol Exp Ther. 2002;301:594–8. doi: 10.1124/jpet.301.2.594. [DOI] [PubMed] [Google Scholar]
- 24.BMC. BMC-2000. 2013. [Last accessed on 2013 Jan 10]. Available from: http://www.senko.co.kr/contents/product/product19.html?sm=3_19 .
- 25.CEBM C. Centre for Evidence Based Medicine Toronto. [Last accessed on 2013 Mar 5]. Available from: http://www.ktclearinghouse.ca/cebm/practise/ca/calculators/statscalc .
- 26.Koren G. Pharmacokinetics in pregnancy; clinical significance. J Popul Ther Clin Pharmacol. 2011;18:e523–7. [PubMed] [Google Scholar]
- 27.Ashford KB, Hahn E, Hall L, Rayens MK, Noland M, Collins R. Measuring prenatal secondhand smoke exposure in mother-baby couplets. Nicotine Tob Res. 2010;12:127–35. doi: 10.1093/ntr/ntp185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin PL, Huang HL, Lu KY, Chen T, Lin WT, Lee CH, et al. Second-hand smoke exposure and the factors associated with avoidance behavior among the mothers of pre-school children: A school-based cross-sectional study. BMC Public Health. 2010;10:606. doi: 10.1186/1471-2458-10-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahluwalia IB, Grummer-Strawn L, Scanlon KS. Exposure to environmental tobacco smoke and birth outcome: Increased effects on pregnant women aged 30 years or older. Am J Epidemiol. 1997;146:42–7. doi: 10.1093/oxfordjournals.aje.a009190. [DOI] [PubMed] [Google Scholar]
- 30.Wahabi HA, Alzeidan RA, Fayed AA, Mandil A, Al-Shaikh G, Esmaeil SA. Effects of secondhand smoke on the birth weight of term infants and the demographic profile of Saudi exposed women. BMC Public Health. 2013;13:341. doi: 10.1186/1471-2458-13-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blake SM, Murray KD, El-Khorazaty MN, Gantz MG, Kiely M, Best D, et al. Environmental tobacco smoke avoidance among pregnant African-American nonsmokers. Am J Prev Med. 2009;36:225–34. doi: 10.1016/j.amepre.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li MF, Wang RH. Factors related to avoidance of environmental tobacco smoke among adolescents in southern Taiwan. J Nurs Res. 2006;14:103–12. doi: 10.1097/01.jnr.0000387568.41941.f0. [DOI] [PubMed] [Google Scholar]
- 33.Kurtz ME, Kurtz JC, Johnson SM, Beverly EE. Exposure to environmental tobacco smoke – Perceptions of African American children and adolescents. Prev Med. 1996;25:286–92. doi: 10.1006/pmed.1996.0058. [DOI] [PubMed] [Google Scholar]
- 34.Wang WL, Herting JR, Tung YY. Adolescents’ avoidance of secondhand smoke exposure: Model testing. West J Nurs Res. 2008;30:836–51. doi: 10.1177/0193945908319251. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Klebanoff MA, Levine RJ, Puri M, Moyer P. The puzzling association between smoking and hypertension during pregnancy. Am J Obstet Gynecol. 1999;181:1407–13. doi: 10.1016/s0002-9378(99)70384-4. [DOI] [PubMed] [Google Scholar]
- 36.Lindqvist PG, Marsál K. Moderate smoking during pregnancy is associated with a reduced risk of preeclampsia. Acta Obstet Gynecol Scand. 1999;78:693–7. [PubMed] [Google Scholar]
- 37.Windsor RA, Warner KE, Cutter GR. A cost-effectiveness analysis of self-help smoking cessation methods for pregnant women. Public Health Rep. 1988;103:83–8. [PMC free article] [PubMed] [Google Scholar]
- 38.Kazemi A, Ehsanpour S, Nekoei-Zahraei NS. A randomized trial to promote health belief and to reduce environmental tobacco smoke exposure in pregnant women. Health Educ Res. 2012;27:151–9. doi: 10.1093/her/cyr102. [DOI] [PubMed] [Google Scholar]
- 39.WHO Report. WHO Report on the Global Tobacco Epidemic. 2011. [Last accessed on 2013 Mar 15]. Available from: http://www.whqlibdoc.who.int/publications/2011/9789240687813_eng.pdf .
- 40.Banning Smoking. Saudi Arabia bans smoking in most public places. 2012. [Last accessed on 2013 Mar 21]. Available from: http://www.bigstory.ap.org/article/saudi-arabia-bans-smoking-most-public-places .


