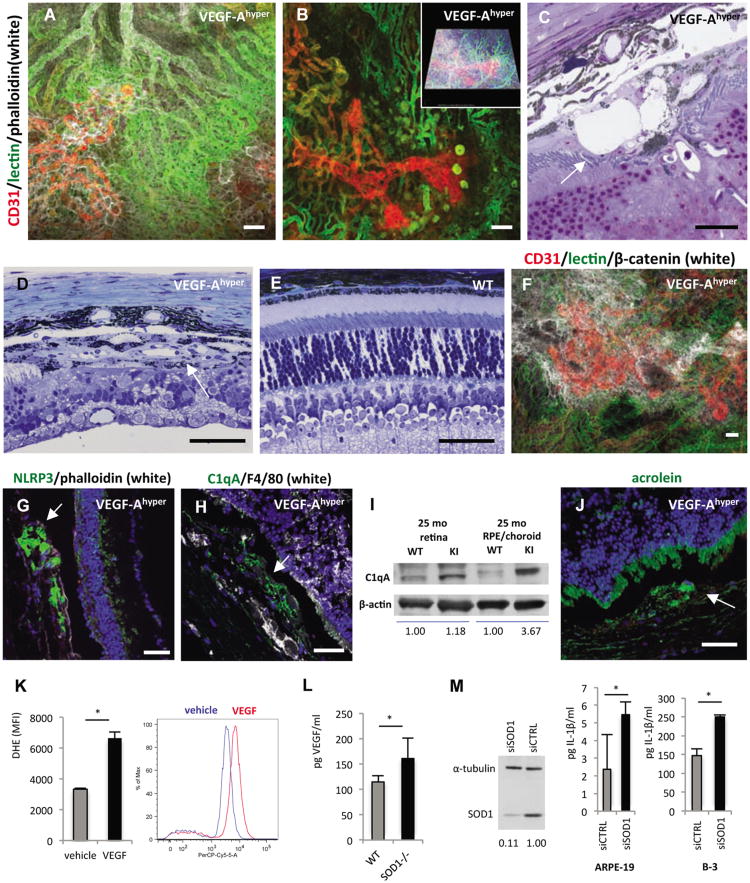
Figure 3. Choroidal neovascularization and NLRP3 expression in aged VEGF-Ahypermice.
a.-b. Perfusion experiments in aged white VEGF-Ahyper mice with fluorescein-conjugated tomato lectin and subsequent wholemount staining for CD31 and phalloidin shows that proliferating neovessels (CD31, red) originate from the underlying perfused choroidal vasculature (green) and extend into the sub-RPE space. Autofluorescent round deposits can be seen at sites of CNV lesions. Scale bars 50μm. Inset in b. represents modeled z-stack of lesion.
c. Representative section of CNV lesion in aged VEGF-Ahyper mice shows massive subretinalneovascularization (arrow). Scale bar 20μm.
d. Representative image of a fully formed CNV lesion in a 7 months old VEGF-Ahyper mouse thatresembles a neovascular membrane in neovascular AMD (arrow). Neovascularization and fibrosishave completely replaced the photoreceptor layer at the site of CNV formation. Scale bar 50μm.
e. In age-matched control littermate mice such lesions were not seen. Scale bar 50μm.
f. Co-localization of RPE barrier breakdown with CNV lesions. Choroidal flatmount of a 24 monthsold white VEGF-Ahyper mouse in which choroidal perfusion is assessed by intracardiac administrationof a fluorescein-conjugated tomato lectin (green). Subsequent whole-mount staining for CD31 (red)highlights proliferating neovessels from underlying perfused choroidal vessels (green), and labelingfor β-catenin (white) shows RPE barrier breakdown at sites of neovascularization with cytoplasmicaccumulation of β-catenin. Scale bar 50μm.
g. Strongly increased NLRP3 expression in RPE cells in CNV lesions (arrows) with attenuation or lossof the overlying photoreceptor layer. 15 months old VEGF-Ahyper mice. Scale bars 50μm.
h. Perivascular accumulation of C1qA within CNV lesions (arrow). 15 months old VEGF-Ahyper mice. Scale bar 50μm.
i. Western blotting demonstrates accumulation of C1qA in choroid/RPE tissues of aged VEGF-Ahyper mice, while control littermate mice showed no accumulation of C1qA. Choroid/RPE or retinal tissues represent pools from three 25 months old VEGF-Ahyper mice or control mice. Normalized relative densitometric values are indicated.
j. Acrolein, a marker for ROS-induced lipid peroxidation, is increased focally in abnormal RPE cells in aged VEGF-Ahyper mice (green, arrow) (15 months old). DAPI nuclear staining; photoreceptor outer segments show autofluorescence (green). Scale bar 50μm.
k. VEGF-A165 treatment of ARPE-19 cells loaded with 10 μM dihydroethidium (DHE) induces increased oxidative stress (superoxide indicator), measured by FACS (PerCP-Cy5-5 channel). Y-axis shows mean fluorescence intensity (MFI) of triplicate experiments (n=3/group). A representative histogram demonstrates the shift of fluorescence induced by VEGF-A165, representing increased superoxide species. *P-value <0.05.
l. VEGF-A serum levels are increased in young (7 weeks old) SOD1-/- mice, compared to age- and gender-matched control littermate mice (n=5). *P-value <0.05.
m. SOD1 is efficiently depleted using SOD1-targeted siRNA, demonstrated by Western blotting of ARPE-19 cell lysate. Cell culture supernatant of cells, primed with 4ng/ml IL-1α, shows that SOD1 knockdown increases levels of the inflammasome effector cytokine IL-1β in both ARPE-19 cells and the B-3 lens epithelial cells. N=4/group. *P-value <0.05. See also Figure S3.