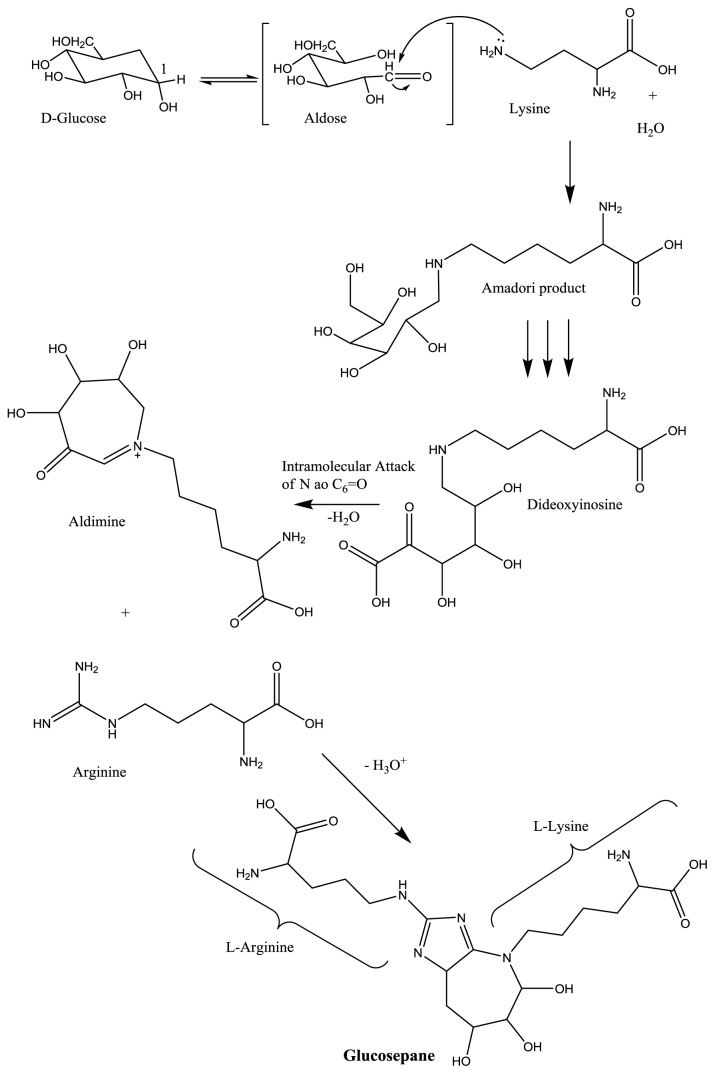
Figure 3.
AGEs formation from reducing sugars. Nucleophilic attack on the anomeric carbon of the sugar by Lys present in proteins, forming Amadori product. Metabolic changes that may cause increased Amadori products are hyperglycemia (Maillard reaction), hipertriglyciridemia and increased fat deposits tissue (lipid peroxidation with formation of reactive carbonyl and dicarbonyl compounds), common processes in obese and type 2 diabetics. After successive displacements of the carbonyl group along the carbon skeleton of the sugar, the intermediate α-dicarbonyl dideoxyinosine is formed. The α-dicarbonyl intermediate suffers intramolecular nucleophilic attack on the carbonyl group by the ɛN, giving aldimine, a precursor for plausible cross-linking agent Lys-Arg, glucosepane. Adapted from [9,17]. C-1, Anomeric carbon on sugar; ɛN, terminal nitrogen of lysine; Lys, lysine; Arg, arginine.