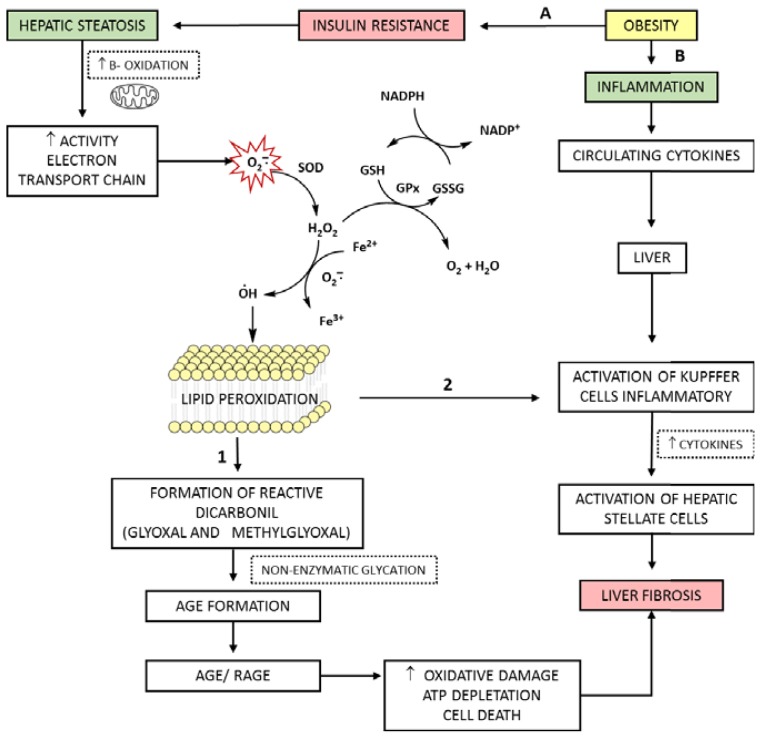
Figure 7.
Evolution of hepatic steatosis to steatohepatitis: Role of AGEs and oxidative stress—a vicious cycle. Obesity is associated with IR (A) and inflammation (B). (A): The IR triggers metabolic changes responsible for the development of hepatic steatosis. Fat oxidation is increased, with higher beta-oxidation reaction and mitochondrial activity. The increased activity in the electron transport chain leads to mitochondrial dysfunction with subsequent formation of ROS; the •O2− formed can be reduced by enzymatic systems to H2O2 (SOD) and by subsequent reaction (GPx) to water; however its reaction with transition metals such as Fe2+ can lead to the formation of the •OH, with subsequent attack to the cell membrane, resulting in lipid peroxidation (LP) in intracellular organelles. Lipid peroxidation: (1) Source of reactive dicarbonyl compounds (reaction intermediates of glycation) which form AGEs (2) Trigger the activation of the inflammatory system, and spread via increased OS. Interaction AGEs/RAGE increases ROS formation, these mechanisms lead the damage oxidative, ATP depletion, cell death, fibrosis and progression of liver disease; (B) Inflammatory cytokines produced by adipose tissue are directed to the liver, strengthening the activation of Kupffer cells inflammatory together the ROS with activation of hepatic stellate cells, contributed to the worsening of fibrosis. SOD, superoxide dismutase; GPx, Glutathione peroxidase; GSH, reduced glutathione; GSSG, oxidized glutathione; NADP+, oxidized nicotinamide adenine dinucleotide phosphate; NADPH, reduced nicotinamide adenine dinucleotide phosphate; Fe2+, ferrous iron; Fe3+, ferric iron; AGEs, advanced glycation end products; RAGE, receptor of advanced glycation end products; ATP, adenosine triphosphate; TNF-α, tumor necrosis factor alpha; IL-6, Interleukin 6; IL-8, Interleukin 8.