Abstract
Objectives
This study examines the relationship between depressive symptoms and walking behavior across 30 months in a prospective study of 217 community-dwelling, Hispanic older adults in Miami, Florida (ages 70–100 years).
Method
Analyses examine the direction of the relationship between depressive symptoms and physical activity (i.e., walking) over time, as well as test for a potential bi-directional or reciprocal relationship between these two variables.
Results
Structural equation modeling (SEM) with a cross-lagged panel design revealed that walking was unrelated to subsequent depressive symptoms. However, depressive symptoms were related to subsequent walking behavior at every time-point, such that higher levels of depressive symptoms were predictive of less walking in the future. Older adults who had clinically-relevant depressive symptoms at the initial assessment had 1.34 times the risk of not walking 30 months later, compared to older adults without clinically-relevant depressive symptoms.
Conclusion
Results support the need for primary care providers to evaluate and address depressive symptoms among older adults, as a means of reducing sedentary behavior and potentially improving health. Further research on the prevention and management of depressive symptoms and sedentary behavior is needed, given the morbidity related to both of these health risks, particularly for minority and low-socio-economic status (SES) older adults.
Keywords: older adults, Hispanics/Latinos, depressive symptoms, physical activity, longitudinal analyses
Introduction
The relationship between depression and physical activity has received substantial attention in the last two decades, in particular among adult and aging populations. Several cross-sectional studies of older adults have documented a significant association between physical inactivity and depressive symptoms (Krause, Goldenhar, Liang, Jay, & Maeda, 1993; Kritz-Silverstein, Barrett-Connor, & Corbeau, 2001; Penninx, Leveille, Ferrucci, van Eijk, & Guralnik, 1999). Despite differences in research methods, measures, and samples, lower levels of physical activity have been associated with higher levels of depressive symptoms fairly consistently across studies (cf, Dunn, Trivedi, & O'Neal, 2001).
Understanding the mechanisms by which depression and physical inactivity are related is important because both are consequential risk factors for some of today's most prevalent chronic illnesses, for instance cardiovascular disease (Bassuk & Manson, 2005; Rugulies, 2002). Depression and physical inactivity also present risks for disability and mortality among older adults (Hirvensalo, Rantanen, & Heikkinen, 2000; Reynolds, Haley, & Kozlenko, 2008). In fact, depression is now considered the leading cause of disability in developed countries (Lopez, Mathers, Ezzati, Jamison, & Maurray, 2006). Longitudinal studies have the potential to understand the mechanisms in operation by uncovering the direction of the relationship between these two variables. Specifically, they may help clarify whether: (1) physical activity improves future depressive symptoms; (2) whether depressive symptoms present barriers to subsequent physical activity; or (3) whether both variables influence each other across time.
However, results from existing longitudinal studies of adult and aging samples have not always been consistent. Some studies have found no prospective link between physical activity and reduced depression (Cooper-Patrick, Ford, Mead, Chang, & Klag, 1997; Kritz-Silverstein et al., 2001; Lennox, Bedell, & Stone, 1990). Other studies have found that low or non-regular levels of physical activity predict higher risk of future depressive symptoms (Camacho, Roberts, Lazarus, Kaplan, & Cohen, 1991; Lampinen, Heikkinen, & Ruoppila, 2000; Morgan & Bath, 1998).
While cross-study comparisons are difficult, study sample differences may be partly responsible for the discrepancies in outcomes. For example, some studies have assessed European samples with less economic hardship, better access to health care, and/or greater physical activity levels than many US subgroups (Lampinen et al., 2000; Morgan & Bath, 1998). Other studies have had few or no depressed individuals in their samples (Camacho et al., 1991; Lampinen et al., 2000). Sometimes this has been by design given concerns that more depressed individuals are inherently less likely to engage in exercise, thereby interfering with the probability of observing an effect of physical activity on subsequent depressive symptoms. Indeed, individuals with depression tend to engage in less physical activity than those without depression (Paluska & Schwenk, 2000). Similarly, depressed older adults have poorer attendance at physical activity programs, and may be less likely to obtain the benefits of these interventions (Nguyen, Koepsell, Unutzer, Larson, & LoGerfo, 2008). Depressive symptoms of diminished interest and fatigue can interfere with physical activity and other activities of daily living (e.g., Crane, 2005; Machado, Gignac, & Badley, 2008; Satariano, Haight, & Tager, 2000). It is important to determine whether the findings of these studies apply to other subgroups of older adults in the US, particularly minority and poor older adults.
A review of the literature on older adults suggests that no study has examined simultaneously the impact of: (1) depressive symptoms on physical inactivity; (2) physical inactivity on depressive symptoms; and (3) the potential bi-directional relationship between depressive symptoms and physical inactivity. This is a particularly important analysis for certain vulnerable subgroups of older adults in the US. One such group is Hispanic, community-dwelling older adults, who tend to have high rates of both clinically-relevant depressive symptoms (Black, Markides, & Miller, 1998; Falcón & Tucker, 2000) and sedentary behaviors (Crespo, Ainsworth, Keteyian, Heath, & Smit, 1999). This group's socio-economic need also places them at risk for health and mental health problems (Mendes de Leon, Rapp, & Kasl, 1994; Perrino, Brown, Mason, & Szapocznik, 2009). Another group at special risk is the ‘old old’ (75–84-year-olds) or ‘oldest old’ (ages 85 years and older), who tend to engage in less physical activity than the ‘younger old’ (Kaplan, Lazarus, Coen, & Leu, 1991). Moreover, a better understanding of the relationship between depressive symptoms and physical inactivity may highlight one of the mechanisms by which some subgroups demonstrate disparities in health problems related to sedentary lifestyles, such as obesity, diabetes, and heart disease.
This study examines the longitudinal relationship between depressive symptoms and physical activity among a population-based sample of Hispanic older adults residing in a poor neighborhood in Miami, Florida. It tests specifically for the direction of the relationship between depressive symptoms and physical activity, as well as whether a reciprocal or bi-directional relationship exists between these variables over time. This is possible by utilizing a cross-lagged panel design and data collected across three points in time. It adds to the existing literature by testing for a more dynamic relationship between these two important variables, and focusing on low-income Hispanic older adults aged 70–100 years, who are at especially high risk of depression and physical inactivity. Finally, it focuses on walking, which is the most common form of physical activity among older adults (USDHHS, 1996).
Methods
Participants
Data were collected as part of a larger, population-based, prospective cohort study of Hispanic older adults in Miami, Florida, entitled ‘The Hispanic Elders’ Behavioral Health Study', which focuses on the relationship between neighborhood environmental factors and older adults' well-being, in particular the built (physical) environment and residents' mental health outcomes. To obtain a population-based sample, all 16,000 households in a single urban Miami community were enumerated to identify all Hispanic adults 70 years or older. One Hispanic older adult was randomly selected from each block on which at least one older adult lived. Of all the 3,322 older adults enumerated, 521 were randomly chosen and approached for participation. To participate, individuals at the baseline had to: (1) be 70 years of age or older, (2) have immigrated from a Spanish-speaking country, (3) reside in this specific Miami neighborhood, (4) reside in housing in which s/he could walk outside (excluding nursing homes), (5) be of sufficient physical health to go outside; and (6) score 17 or above on the Mini-Mental State Examination, or MMSE (Folstein, Folstein, & McHugh, 1975), a test of global cognitive functioning. Of the 521 initially approached, 30 had died since enumeration, 87 had moved or could not be located/contacted, 95 refused participation, 10 had incorrect home addresses, 24 did not meet full eligibility criteria, and 2 moved to a different block from which a participant had already been sampled, resulting in 273 participants who provided informed consent and completed the baseline assessment.
Participants completed yearly, home-based assessments in Spanish at baseline, 12-, 24-, 36-, and 54-month follow-ups. Information regarding older adults' walking patterns was collected starting at the 24-month assessment (the third wave of data from the larger study). Consequently, data for this specific study were collected during 24-month, 36-month, and 54-month assessments. By the 24-month assessment, the original sample of 273 older adults had been reduced to 217 due to death (N = 25), refusal (N = 11), moving out of the region (N = 7), and loss to follow-up (N = 13). For clarity, the three assessments used in this study will be referred to throughout the remainder of this article as the Initial Assessment (24-month assessment), Follow-Up 1 (36-month assessment), and Follow-Up 2 (54-month assessment). References to ‘baseline’ will continue to refer to the ‘true’ start of the larger study.
Baseline assessments were completed between 2002 and 2004. At baseline, the mean age was 78.5 years (SD = 6.3; range = 70–100). Of these 59% were female. Approximately 86% were Cuban-born or relocated to Cuba as children and 10% were born in a Central or South American country. Participants had lived in the US for an average of 29 years. The sample was composed primarily of individuals of low socio-economic status (SES), with 71% reporting annual household income less than $10,000, and most reporting working class jobs prior to retirement (e.g., factory worker, housekeeper). The sample averaged 7.3 years of education (SD = 4.3; range = 0–20 years). Of the participants, 34% were married, 35% widowed, 20% separated or divorced, and 11% never married.
Measures
Depressive symptoms
A Spanish translation of the 20-item Center for Epidemiologic Studies Depression Scale, or CES-D (Radloff, 1977), was used to assess self-reported depressive symptoms in the preceding week. A continuous total sum score was computed, such that higher scores indicated more depressive symptoms. The CES-D has demonstrated good test–retest reliability and has been used successfully with Hispanic and Spanish-speaking older adults (Black et al., 1999). At baseline, Cronbach's α for the CES-D in this sample was 0.86.
Physical activity (walking)
Participants were asked to report their walking routes during the preceding 7 days using the timeline follow-back technique (Sobell & Sobell, 1996), an established interview method that uses a calendar as a cue to remind the respondent of life events in the last week. The events are recorded on the calendar to help recall before asking for target behaviors. In this method, the assessor begins by producing a 7-day calendar form and placing the day of the week for each of the 7 days, asking the participant to recall events that have occurred in any of the past 7 days including destinations outside the home (e.g., trip to the doctor, friend's birthday). Next, the assessor shows the calendar form to the older adults, along with a large map of the neighborhood. The calendar is used to cue the participant's memory for her/his behavior in the last 7 days that might be associated with walking (e.g., walking to a friend's house for a birthday). Then the assessor shows the participant where s/he lives on the map and asks the participant where s/he walked on for each day's walking trip and what route s/he took to get there. The assessor traces the actual route walked on the map and notes the specific blocks walked. This process is repeated for the return route, including a note of any transportation that the older adult might have taken on either the initial or return trips (e.g., car, bus, taxi). Data are transformed into a specific identification number assigned to that block, and the number of blocks that are walked are then summed to obtain the ‘total blocks walked’.
Control variables were based on values at the 24-month assessment (the Initial Assessment for this study), and included: gender, age, marital disruption (divorce, death, separation versus never married, married), years of education, financial strain, body mass index (BMI), and health status. Financial strain was assessed using a single item about how difficult it was to pay for basic needs using a response scale ranging from 1 (‘Easy’) to 7 (‘Very difficult’). Health status was assessed using two items, each of which asks participants to rate their health ‘at the present time’ and ‘in general’ using a five-point scale from 1 (‘Excellent’) to 5 (‘Poor’). These perceived global health items have been significantly associated with functional abilities (Benyamini, Idler, Leventhal, & Leventhal, 2000; Idler & Kasl, 1995).
Analytic model
Preliminary analyses examined variable distributions, sample characteristics, and attrition. Primary analyses used Amos 7 (SPSS, 2006) and focused on structural equation modeling (SEM). A cross-lagged panel design was used to test the relationships between depressive symptoms and blocks walked across the three time points. Analyses included age, gender, education, marital disruptions, financial strain, BMI, and health status at the Initial Assessment (the 24-month assessment in the larger project) as control variables. Among the strengths of using a cross-lagged panel analysis approach is that it allows simultaneous analysis of the two dependent variables, thereby permitting the identification of possible bi-directional associations between depressive symptoms and blocks walked across time. In addition, post hoc analyses examined the same model among those older adults in the Initial Assessment dataset who continued to be alive at Follow-up 2 (the 54-month assessment in the larger project), as well as a binomial regression predicting walking-status at Follow-up 2.
The SEM analyses involved developing an initial ‘Full Model’ in which all paths were estimated, including both sets of cross-lagged paths between depressive symptoms and blocks walked. In the Full Model, all control variables were allowed to correlate with each other, and all control variables served as potential predictors of both depression scores and blocks walked at the Initial Assessment. Furthermore, within each of the pair of cross-lagged effects, the two paths were constrained to be equal. This was done in order to provide a more conservative test of these effects. Specifically, given that this is a non-experimental design in which different participants are of different ages, there was no reason to believe that the cross-lagged effects would consistently differ in a systematic pattern from one assessment to the next across all participants (i.e., on average, the cross-lagged effect from the Initial Assessment to Follow-up 1 should be about the same as the cross-lagged effect from Follow-up 1 to Follow-up 2).
In addition to lag 1 autocorrelations between subsequent assessments of depression and blocks walked, lag 2 autocorrelation effects were also included to allow for more cyclical patterns of effect and/or differential short- and long-term effects (see, e.g., Shahar & Davidson, 2003, for a similar approach). This consisted of a path from depressive symptoms at the Initial Assessment to depressive symptoms at Follow-up 2, and from blocks walked at the Initial Assessment to blocks walked at Follow-up 2. To illustrate how this would address cyclical patterns and/or differential short- and long-term effects, consider how this would influence predicted depressive symptoms at Follow-up 2 for a person who fluctuated from below-average depressive symptoms scores at the Initial Assessment to above-average depressive symptoms scores at Follow-up 1. A model with only a single lag path from Follow-up 1 depressive symptoms to Follow-up 2 depressive symptoms would predict relatively high levels of depressive symptoms at Follow-up 2 because the predicted score would only reflect the high levels of depressive symptoms at Follow-up 1. In contrast, a model with an additional lag path from Initial Assessment depressive symptoms to Follow-up 2 depressive symptoms would predict Follow-up 2 depressive symptoms scores closer to the mean because these predicted scores would reflect both the high levels of depressive symptoms at Follow-up 1 and the historically lower levels of depressive symptoms at the Initial Assessment. In essence, when scores fluctuate in more extreme ways over time, the lag 2 effect balances these fluctuations when predicting scores for subsequent assessments. It should be noted that removing these lag 2 effects dramatically reduced the overall fit of the model, as would be expected if such effects were occurring. Finally, it should be noted that preliminary analyses tested for possible time-varying covariate effects for those control variables whose values may have changed over time; however, these were not statistically significant, and so control variables were included as predictors of depression and blocks walked only at the Initial Assessment.
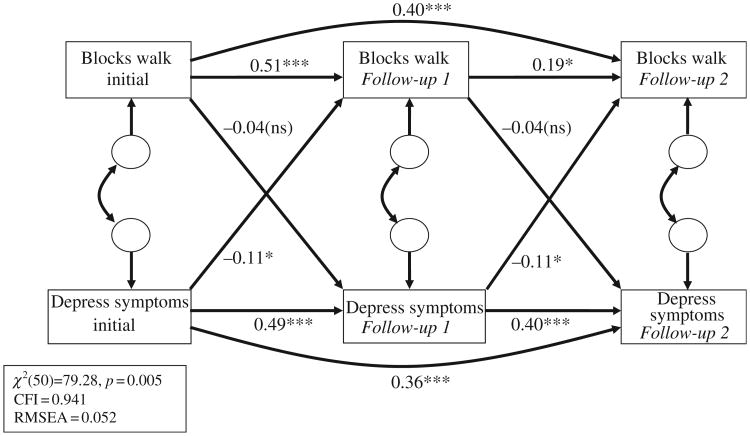
While the Full Model did result in an adequate fit with the data (CFI = 0.928, RMSEA = 0.072), given the modest sample size we sought to minimize the number of parameters being estimated. We did this by eliminating all non-significant paths between the control variables and either depression or blocks walked. We also eliminated all non-significant correlations between the control variables themselves. It should be noted that removal of these paths had no significant impact on the other path coefficients, but given the modest sample size, was done in order to minimize the number of parameters that needed to be estimated. This produced the Final Model, which is presented in Figure 1. To reduce clutter and highlight the cross-lagged pathways which are the focus of this study, path coefficients for the control variables are specified in Table 3 rather than in Figure 1.
Figure 1.
Final model. Control Variables: Difficulty paying for basic necessities, marital disruption, BMI, health status, gender, education, age. Additional Paths: Significant covariances between control variables were also included; significant paths between control variables and initial values of blocks walked and depressive symptoms were also included.
Note: *p < 0.05; ***p < 0.001.
Table 3.
Control variable effects.
| Path | Coefficient | p-value |
|---|---|---|
| Education ≥ Depressive symptoms (Initial Assessment) | −0.16 | p = 0.005 |
| Gender ≥ Blocks walked (Log, Initial Assessment) | −0.16 | p = 0.015 |
| Health status ≥ Blocks walked (Log, Initial Assessment) | 0.14 | p = 0.028 |
| Health status ≥ Depressive symptoms (Initial Assessment) | −0.53 | P < 0.001 |
| Age ≥ Blocks walked (Log, Initial Assessment) | −0.24 | P < 0.001 |
| Education ≥ Blocks walked (Log, Initial Assessment) | −0.12 | p = 0.063 |
| Marital disruption ≥ Depressive symptoms (Initial Assessment) | 0.10 | p = 0.088 |
| BMI ≥ Blocks walked (Log, Initial Assessment) | −0.18 | p = 0.009 |
Results
Attrition/missing data across time-points
As noted previously, participants decreased from 273 at baseline to 217 at the 24-month assessment, which served as the Initial Assessment of the specific substudy described. Attrition up to the 24-month assessment (i.e., the first assessment at which physical activity data was collected) was due to death (N = 25), refusal (N = 11), moving out of the region (N = 7), and loss to follow-up (N = 13). By Follow-up 2 (i.e., the 54-month assessment) which marked the end of the current substudy, enrollment had declined by an additional 53 participants (30 died, 2 were incapacitated, 7 refused, 6 moved out of the region, and 8 were lost to follow-up). Missing data were addressed using a full information maximum likelihood (FIML) algorithm (Arbuckle, 1996).
Descriptive information regarding depressive symptoms and blocks walked
Means and standard deviations of the depressive symptoms scale and blocks walked at each of the three assessments are presented in Table 1. In particular, mean values for depression scores were fairly stable over time. This was the case whether one examined the means based on all data at each time point (as presented in Table 1) or means based on only those individuals who remained in the sample through Follow-up 2. However, while the mean across participants may have been stable from one assessment to the next, scores for individual participants did vary, increasing for some and decreasing for others. On average, this individual variation cancelled out, resulting in the fairly flat, stable sample averages across assessments. Therefore, in order to estimate the degree to which scores for individual participants changed over time, the absolute value of the difference between the Initial Assessment and Follow-up 2 was also calculated. The mean of the absolute change on the depression scale corresponded to 0.64 standard deviations, while the mean of the absolute change in blocks walked corresponded to 0.57 standard deviations. This change could reflect either increasing or decreasing scores, as it was calculated based on the absolute value of the change observed. In other words, while the sample average across all participants did not change over time, the typical participant increased or decreased nearly two-thirds of a standard deviation in depressive symptoms and over one-half of a standard deviation in blocks walked. However, because this change was positive for some and negative for others, the overall mean levels across the entire sample remained fairly flat.
Table 1.
Depressive symptoms and blocks walked descriptive statistics.
| Variable | Mean | Standard deviation | Min. | Max. | N |
|---|---|---|---|---|---|
| Depressive symptoms initial assessment | 11.31 | 9.63 | 0.00 | 46.00 | 214 |
| Depressive symptoms Follow-up 1 | 11.37 | 8.71 | 0.00 | 43.00 | 192 |
| Depressive symptoms Follow-up 2 | 11.37 | 8.03 | 0.00 | 45.00 | 164 |
| Blocks walked Initial Assessment | 24.93 | 40.26 | 0.00 | 302.00 | 215 |
| Blocks walked Follow-up 1 | 22.32 | 37.11 | 0.00 | 208.00 | 194 |
| Blocks walked Follow-up 2 | 16.73 | 28.85 | 0.00 | 186.00 | 164 |
Based on the commonly used CES-D cut-off score of 16 and above (Cho, et al., 1993), clinically elevated levels of depressive symptoms were stable over the course of this study, varying from 27.6% at the Initial Assessment of the current study (i.e., 24-months), 28.6% at Follow-up 1 (i.e., 36-months), and 27.4% at Follow-up 2 (i.e., 54-months). The number of blocks walked was very skewed, with 44.2% of participants walking zero blocks at the Initial Assessment, 53.1% walking zero blocks at Follow-up 1, and 59.8% walking zero blocks at Follow-up 2. Consequently, unless otherwise noted, the number of blocks walked was log transformed (natural logarithm of the sum of the number of blocks walked plus 1) for all subsequent analyses.
Correlation matrix
Table 2 presents the bivariate correlations between variables at the start of the current study. These suggested that higher health ratings were related to lower depressive symptoms and a higher number of blocks walked at the Initial Assessment; while women reported higher levels of depressive symptoms and walked fewer blocks. Similarly, higher education and lower reported financial strain were associated with lower depressive symptoms. Finally, as expected, higher levels of depressive symptoms were related to walking fewer blocks.
Table 2.
Correlation matrix at initial assessment.
| Age | Gender | Education | Marital disruption | Health status | BMI | Financial strain | Depression symptoms | Blocks walked | |
|---|---|---|---|---|---|---|---|---|---|
| Age | 1.000 | ||||||||
| Gender | 0.171* | 1.000 | |||||||
| Education | −0.146* | −0.091 | 1.000 | ||||||
| Marital Disruption | 0.147* | 0.293*** | −0.042 | 1.000 | |||||
| Health status | −0.115 | −0.250*** | 0.055 | −0.026 | 1.000 | ||||
| BMI | −0.293*** | 0.090 | −0.022 | −0.086 | −0.093 | 1.000 | |||
| Financial strain | 0.034 | −0.013 | −0.180** | 0.013 | −0.235** | −0.013 | 1.000 | ||
| Depression symptoms | 0.099 | 0.186** | −0.191** | 0.112 | −0.535*** | −0.039 | 0.216** | 1.000 | |
| Log-blocks walked | −0.210** | −0.243*** | −0.062 | −0.037 | 0.225** | −0.128 | −0.049 | −0.230** | 1.000 |
Note:
p < 0.05;
p < 0.01;
p < 0.001
Model development
Figure 1 shows the Final Model, after eliminating (1) non-significant control variable effects on depressive symptoms and blocks walked and (2) non-significant correlations among control variables. For clarity, control variable effects are not shown in Figure 1; instead, regression weights and p-values for the control variables are shown in Table 3. As reflected in Figure 1, model fit indices showed an adequate fit to the data, specifically: χ2/df = 1.59, CFI = 0.941, and RMSEA = 0.052 (model χ2(50) = 79.28, p = 0.005). As expected, both the immediate autocorrelation effects for both blocks walked and depressive symptoms were significant, as were both of the lag-2 autocorrelation effects. Furthermore, the cross-lagged effects from depressive symptoms to blocks walked were statistically significant, indicating that higher levels of depression at one time point were related to fewer blocks walked in the subsequent future assessment. In contrast, the cross-lagged effects from blocks walked to depressive symptoms were not statistically significant, suggesting that walking did not predict future depressive symptoms.
Post hoc analyses
To determine whether the loss of participants who had died during the study influenced the results, the Full Model was re-analyzed including only the 187 participants at the Initial Assessment who remained alive through Follow-up 2. Again, these results showed a pattern similar to the Final Model based on all participants. Model fit indices continued to show an adequate fit to the data, specifically: χ2/df = 1.44, CFI = 0.953, and RMSEA = 0.049 (model χ2(50) = 71.97, p = 0.023). The cross-lagged paths from blocks walked to depression paths were not statistically significant (i.e., Blocks walked, Initial Assessment → Depression, Follow-up 1 = −0.03, p = 0.43; Blocks walked, Follow-up 1 → Depression, Follow-up 2 = −0.04, p = 0.43), while the cross-lagged paths from depression to blocks walked were significant (i.e., Depression, Initial Assessment → Blocks walked, Follow-up 1 = −0.11, p = 0.02; Depression, Follow-up 1 → Blocks walked, Follow-up 2 = −0.11, p = 0.02).
We conducted a second post hoc analysis to gain additional depth and practical interpretability for the relationship between depressive symptoms and future walking behavior among and within this older adult sample. For this analysis, we performed a binomial regression predicting whether or not an older adult walked or did not walk at Follow-up 2, based on whether he or she reported clinically-elevated levels of depressive symptoms (for CES-D of 16 and above, see Cho et al., 1993) at the Initial Assessment. Controlling for the same set of demographic variables used in the cross-lag analysis – gender, age, education, marital disruption, health status, BMI, and financial strain – clinically elevated depression at the Initial Assessment was significantly related to whether or not an older adult walked at Follow-up 2. Specifically, adjusting for these covariates, the predicted rate of non-walking among those older adult with non-clinically elevated depression scores at the Initial Assessment was 54.8%, while the predicted rate among those with clinically elevated rates of depression at the Initial Assessment was 73.5%. This 18.7% difference was statistically significant [χ2(1) = 4.430; p = 0.035; 95% confidence interval (CI), 0.013–0.361]. This corresponds to a relative risk of 1.34, or an older adult with clinically elevated levels of depression at the Initial Assessment being 1.34 times more likely to not walk at Follow-up 2, relative to an older adult without clinically elevated depression at the Initial Assessment.
Discussion
The study results show that greater depressive symptoms predicted less walking at subsequent assessment time-points. Specifically, for older adults with clinically relevant depressive symptoms at the Initial Assessment, the rate of sedentary behavior (i.e., not walking) at the final follow-up was 74%, while for those not showing clinically relevant symptoms, the rate of sedentary behavior was 55%, a statistically significant difference. These findings are consistent with extant research suggesting that depressive symptoms can present a formidable barrier to physical activity among older adults (Nguyen et al., 2008). Relatedly, depressive symptoms can augment the risk of disability, something that has been at least partly explained by reduced physical activity (Penninx et al., 1999). Indeed, a study that compared different health conditions based upon their impact on functioning found that individuals with depressive symptoms tended to report more limited physical functioning (i.e., more days in bed) than did individuals with hypertension, diabetes, or arthritis (Wells et al., 1989). The latter study further reported that only chest pain and advanced coronary artery disease were more limiting in terms of interfering with daily activities such as walking, dressing, bathing, climbing stairs, or participating in sports. Clinically relevant depressive symptoms that may not reach full criteria for a depressive disorder are important because even subthreshold depression levels have been found to predict disability in older adults (Chopra et al., 2005). Indeed, depressive symptoms and their sequelae will become even more notable public health problems, given that depression is now the leading cause of disability in the developed world (Lopez et al., 2006).
In contrast, this study found that older adults who walked more were no more or less likely to experience depressive symptoms at later time-points than those who walked less. While some researchers have found evidence that higher levels of physical activity are related to fewer subsequent depressive symptoms (Camacho et al., 1991; Lampinen et al., 2000; Morgan & Bath, 1998), this was not the case in this study. There are noteworthy differences in the present study's sample that may have affected these findings. First, as with many US Hispanic older adults (Falcón & Tucker, 2000), this sample demonstrated higher levels of clinically relevant depressive symptoms compared with most other subgroups of US older adults, and their high levels of perceived financial need places them at greater risk for depressive symptoms (Perrino et al., 2009). Past depressive symptoms have been among the strongest predictors of current depressive symptoms, regardless of exercise level (Lampinen et al., 2000; Morgan and Bath, 1998). It may be that this high prevalence of depressive symptoms, intensified by socio-economic need and possibly advanced age, presents a more challenging barrier to physical activity than it does for other groups. Unfortunately, this limits this group's chances of obtaining the protective health benefits that exercise can provide.
Second, and connected to the previous point, this group of older adults exhibited low rates of physical activity in general, with large percentages reporting no walking at all − 44% at the Initial Assessment, and 53% and almost 60% at the subsequent two time-points, respectively. This sample is composed of an ‘older’ sample of older adults (70–100 years old), who may be at greater risk of health problems and impairments, and less likely to engage in physical activity than ‘younger’ older adults (Kaplan et al., 1991). While age and self-reported health were both control variables in the present analyses, it has been argued that certain threshold amounts of physical activity may be necessary to improve mood and/or depressive symptoms (Morgan & Bath, 1998). As a group, the older adults in this sample may not have reached these threshold levels. Nevertheless, low rates of physical activity are common among older adults across the US (Eyler, Brownson, Bacak, & Housemann, 2003; USDHHS, 1996), particularly among Hispanics and other minority groups (Kruger, Ham & Sanker, 2008), as well as individuals of low SES (Yancey et al., 2005). The findings of this study therefore, are likely to reflect the reality of many present-day, disadvantaged older adults.
The impact of depressive symptoms on physical activity, combined with the high prevalence of depressive symptoms and low prevalence of physical activity, may be important in explaining certain health disparities evidenced by Hispanics. For instance, Hispanics have higher rates of hypertension and obesity than non-Hispanic whites, as well as higher mortality rates from diabetes (USDHHS, 2000), all conditions that can be prevented or managed with the help of regular physical activity. Depressive symptoms have also been linked more directly to various health problems, including cardiovascular disease (Bassuk & Manson, 2005; Rugulies, 2002). Further research is needed to clearly understand the sources of these disparities. The fact that Hispanic older adults represent a rapidly growing segment of the US population, suggests this is of large public health significance.
Although the study's physical activity measure assesses walking, the most common form of exercise among older adults (USDHHS, 1996), the measure has certain shortcomings. First, it is not an objective measure, such as pedometers or accelerometers, and only assesses walking in the preceding week. It also does not account for walking pace, which can have a substantial impact of fitness levels. Similarly, another shortcoming of this study is that while self-reported health was a control variable, it was not an objective measure of health, health problems, or disability. This is important because health problems and disability may be quite influential in both depressive symptoms and in the extent of physical activity or walking. Yet, it should be noted that self-reported health has been significantly associated with physical health, functional abilities, and even mortality (Idler, Kasl, & Lemke, 1990; Benyamini et al., 2000; McGee, Liao, Cao, & Cooper, 1999).
This study is unique in that it investigates the relationship between depressive symptoms and physical inactivity, two variables with important health implications for older adults. The utilization of a cross-lagged panel analysis allowed the simultaneous examination of the impact of: depressive symptoms on physical inactivity, physical inactivity on depressive symptoms, and the potential bi-directional relationship between depressive symptoms and physical inactivity. Although the only significant, prospective relationship was between depressive symptoms and physical inactivity, this was significant across all time-points. This finding is especially consequential in this community-dwelling sample of Hispanic older adults given their high levels of clinically relevant depressive symptoms.
The study results support the need for primary care providers and clinicians to recognize and intervene to address depressive symptoms among older adults. Effective interventions could help minimize the negative impact of depression on health and quality of life, by increasing the likelihood of protective health behaviors, such as physical activity. Treatment and improvement of depressive symptoms have been related to improvement in instrumental activities of daily living among older adults (see, e.g., Oslin, Streim, Katz, Edell, & TenHave, 2000; Yochim, Lequerica, MacNeill, & Lichtenberg, 2008), which often include exercise or activities that involve physical activity, such as shopping and housekeeping. Future research should address the identification and treatment of depressive symptoms as a possible means of increasing physical activity and reducing health disparities in Hispanic older adults, a growing segment of the population.
Acknowledgments
This work was supported by a National Institute of Mental Health/National Institute of Environmental Health Sciences Grant No. MH 63709 (J. Szapocznik, PI; A. Spokane, Co-PI), by a National Institute on Aging Grant No. AG 027527 (J. Szapocznik, PI; S. Brown, Co-PI), and by a Robert Wood Johnson Foundation Grant No. 037377. We thank Monica Zarate, Rosa Verdeja, Tatiana Clavijo, Aleyda Marcos, and Patricia Thomas for their help in conducting this study.
References
- Arbuckle JL. Full information estimation in the presence of incomplete data. In: Marcoulides GA, Schumacker RE, editors. Advanced structural equation modeling: Issues and techniques. Mahwah, NJ: Lawrence Erlbaum Associates; 1996. pp. 243–277. [Google Scholar]
- Bassuk SS, Manson JE. Epidemiological evidence for the role of physical activity in reducing risk of type 2 diabetes and cardiovascular disease. Journal of Applied Physiology. 2005;99(3):1193–1204. doi: 10.1152/japplphysiol.00160.2005. [DOI] [PubMed] [Google Scholar]
- Benyamini Y, Idler EL, Leventhal H, Leventhal EA. Positive affect and function as Influences on self-assessments of health: Expanding our view beyond illness and disability. Journal of Gerontology: Psychological Sciences. 2000;55B:P107–P116. doi: 10.1093/geronb/55.2.p107. [DOI] [PubMed] [Google Scholar]
- Black SA, Espino DV, Mahurin R, Lichtenstein MJ, Hazuda HP, Fabrizio D, et al. The influence of noncognitive factors on the Mini-Mental State Examination in older Mexican Americans: Findings from the Hispanic EPESE-Established Populations for the Epidemiologic Study of the Elderly. Journal of Clinical Epidemiology. 1999;52(11):1095–1102. doi: 10.1016/s0895-4356(99)00100-6. [DOI] [PubMed] [Google Scholar]
- Black SA, Markides KS, Miller TQ. Correlates of depressive symptomatology among older community-dwelling Mexican Americans: The Hispanic EPESE. Journal of Gerontology: Social Sciences. 1998;53B(4):S198–S208. doi: 10.1093/geronb/53b.4.s198. [DOI] [PubMed] [Google Scholar]
- Camacho TC, Roberts R, Lazarus NB, Kaplan GA, Cohen RD. Physical activity and depression: Evidence from the Alameda County Study. American Journal of Epidemiology. 1991;134(2):220–231. doi: 10.1093/oxfordjournals.aje.a116074. [DOI] [PubMed] [Google Scholar]
- Cho MJ, Moscicki EK, Narrow WE, Rae DS, Locke BZ, Regier DA. Concordance between two measures of depression in the Hispanic Health and Nutrition Examination Survey. Social Psychiatry and Psychiatric Epidemiology. 1993;28:156–163. doi: 10.1007/BF00797317. [DOI] [PubMed] [Google Scholar]
- Chopra MP, Zubritsky C, Knott K, Have TT, Hadley T, Coyne JC, et al. Importance of subsyndromal symptoms of depression in elderly patients. Archives of Geriatric Psychiatry. 2005;13(7):597–606. doi: 10.1176/appi.ajgp.13.7.597. [DOI] [PubMed] [Google Scholar]
- Cooper-Patrick L, Ford DE, Mead LA, Chang PP, Klag MJ. Exercise and depression in mid-life: A prospective study. American Journal of Public Health. 1997;87(4):670–673. doi: 10.2105/ajph.87.4.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane PB. Fatigue and physical activity in older women after myocardial infarction. Heart & Lung: The Journal of Critical Care. 2005;34(1):30–38. doi: 10.1016/j.hrtlng.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Crespo CJ, Ainsworth BE, Keteyian SJ, Heath GW, Smit E. Prevalence of physical inactivity and its relation to social class in US adults: Results from the Third National Health and Nutrition Examination Survey, 1988–1994. Medicine and Science in Sports and Exercise. 1999;31(12):1821–1827. doi: 10.1097/00005768-199912000-00019. [DOI] [PubMed] [Google Scholar]
- Dunn AL, Trivedi MH, O'Neal HA. Physical activity dose–response effects on outcomes of depression and anxiety. Medicine and Science in Sports and Exercise. 2001;33(6):S587–S597. doi: 10.1097/00005768-200106001-00027. [DOI] [PubMed] [Google Scholar]
- Eyler AA, Brownson RC, Bacak SJ, Housemann RA. The epidemiology of walking for physical activity in the United States. Medicine and Science in Sports and Exercise. 2003;35(9):1529–1536. doi: 10.1249/01.MSS.0000084622.39122.0C. [DOI] [PubMed] [Google Scholar]
- Falcón LM, Tucker KL. Prevalence and correlates of depressive symptoms among Hispanic elders in Massachusetts. Journal of Gerontology: Social Sciences. 2000;55B(2):S108–S116. doi: 10.1093/geronb/55.2.s108. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Hirvensalo M, Rantanen T, Heikkinen E. Mobility difficulties and physical activity as predictors of mortality and loss of independence in the community-living older population. Journal of the American Geriatric Society. 2000;48(5):493–498. doi: 10.1111/j.1532-5415.2000.tb04994.x. [DOI] [PubMed] [Google Scholar]
- Idler EL, Kasl SV. Self-ratings of health: Do they also predict change in functional ability? The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 1995;50(6):S344–S353. doi: 10.1093/geronb/50b.6.s344. [DOI] [PubMed] [Google Scholar]
- Idler EL, Kasl SV, Lemke JH. Self-evaluated health and mortality among the elderly in New Haven, Connecticut and Iowa and Washington Counties, Iowa, 1982–1986. American Journal of Epidemiology. 1990;131(1):91–103. doi: 10.1093/oxfordjournals.aje.a115489. [DOI] [PubMed] [Google Scholar]
- Kaplan GA, Lazarus NB, Coen RD, Leu DJ. Psychosocial factors in the natural history of physical activity. American Journal of Preventive Medicine. 1991;7:12–17. [PubMed] [Google Scholar]
- Krause N, Goldenhar L, Liang J, Jay G, Maeda D. Stress and exercise among the Japanese elderly. Social Science and Medicine. 1993;36:1429–1441. doi: 10.1016/0277-9536(93)90385-h. [DOI] [PubMed] [Google Scholar]
- Kritz-Silverstein D, Barrett-Connor E, Corbeau C. Cross-sectional and prospective study of exercise and depressed mood in the elderly. American Journal of Epidemiology. 2001;153(6):596–602. doi: 10.1093/aje/153.6.596. [DOI] [PubMed] [Google Scholar]
- Kruger J, Ham SA, Sanker S. Physical inactivity during leisure time among older adults – Behavioral Risk Factor Surveillance System, 2005. Journal of Aging & Physical Activity. 2008;16(3):280–291. doi: 10.1123/japa.16.3.280. [DOI] [PubMed] [Google Scholar]
- Lampinen P, Heikkinen RL, Ruoppila I. Changes in intensity of physical exercise as predictors of depressive symptoms among older adults: An eight-year follow-up. Preventive Medicine: An International Journal Devoted to Practice and Theory. 2000;30(5):371–380. doi: 10.1006/pmed.2000.0641. [DOI] [PubMed] [Google Scholar]
- Lennox SS, Bedell JR, Stone AA. The effect of exercise on normal mood. Journal of Psychosomatic Research. 1990;34(6):629–636. doi: 10.1016/0022-3999(90)90106-e. [DOI] [PubMed] [Google Scholar]
- Lopez AD, Mathers CD, Ezzati M, Jamison D, Maurray CJL, editors. Global burden of diseases and risk factors. New York: Oxford University Press; 2006. Measuring the global burden of disease and risk factors, 1990–2001. [PubMed] [Google Scholar]
- Machado GPM, Gignac MAM, Badley EM. Participation restrictions among older adults with osteoarthritis: A mediated model of physical symptoms, activity limitations, and depression. Arthritis & Rheumatism (Arthritis Care & Research) 2008;59(1):129–135. doi: 10.1002/art.23259. [DOI] [PubMed] [Google Scholar]
- McGee DL, Liao Y, Cao G, Cooper RS. Self-reported health status and mortality in a multiethnic US cohort. American Journal of Epidemiology. 1999;149(1):41–46. doi: 10.1093/oxfordjournals.aje.a009725. [DOI] [PubMed] [Google Scholar]
- Mendes De, Leon CF, Rapp SS, Kasl SV. Financial strain and symptoms of depression in a community sample of elderly men and women: A longitudinal study. Journal of Aging and Health. 1994;6(4):448–468. [Google Scholar]
- Morgan K, Bath PA. Customary physical activity and psychological well-being: A longitudinal study. Age and Ageing. 1998;27:35–40. doi: 10.1093/ageing/27.suppl_3.35. [DOI] [PubMed] [Google Scholar]
- Nguyen HQ, Koepsell T, Unutzer J, Larson E, LoGerfo JP. Depression and use of a health plan sponsored physical activity program by older adults. American Journal of Preventive Medicine. 2008;35(2):111–117. doi: 10.1016/j.amepre.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oslin DW, Streim J, Katz IR, Edell WS, TenHave T. Change in disability follows inpatient treatment for late life depression. Journal of the American Geriatrics Society. 2000;48:357–362. doi: 10.1111/j.1532-5415.2000.tb04690.x. [DOI] [PubMed] [Google Scholar]
- Paluska SA, Schwenk TL. Physical activity and mental health: Current concepts. Sports Medicine. 2000;29(3):167–180. doi: 10.2165/00007256-200029030-00003. [DOI] [PubMed] [Google Scholar]
- Penninx BWJH, Leveille S, Ferrucci L, van Eijk JTM, Guralnik JM. Exploring the effect of depression on physical disability: Longitudinal evidence from the established populations for epidemiologic studies of the elderly. American Journal of Public Health. 1999;89(9):1346–1352. doi: 10.2105/ajph.89.9.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrino T, Brown SC, Mason CA, Szapocznik J. Depressive symptoms among urban Hispanic older adults in Miami: Prevalence and sociodemographic correlates. Clinical Gerontologist. 2009;32:26–42. doi: 10.1080/07317110802478024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- Reynolds SL, Haley WE, Kozlenko N. The impact of depressive symptoms and chronic diseases on active life expectancy in older Americans. American Journal of Geriatric Psychiatry. 2008;16(5):425–432. doi: 10.1097/JGP.0b013e31816ff32e. [DOI] [PubMed] [Google Scholar]
- Rugulies R. Depression as a predictor of coronary heart disease: A review and meta-analysis. American Journal of Preventive Medicine. 2002;23(1):51–61. doi: 10.1016/s0749-3797(02)00439-7. [DOI] [PubMed] [Google Scholar]
- Satariano WA, Haight TJ, Tager IB. Reasons given by older people for limitation or avoidance of leisure time physical activity. Journal of the American Geriatrics Society. 2000;48(5):505–512. doi: 10.1111/j.1532-5415.2000.tb04996.x. [DOI] [PubMed] [Google Scholar]
- Shahar G, Davidson L. Depressive symptoms erode self-esteem in severe mental illness: A three-wave, cross-lagged study. Journal of Consulting and Clinical Psychology. 2003;31(5):890–900. doi: 10.1037/0022-006X.71.5.890. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Followback User's Guide: A calendar method for assessing alcohol and drug abuse. Toronto, Canada: Addiction Research Foundation; 1996. [Google Scholar]
- SPSS, Inc. Amos 7.0: Structural equation modeling to test relationships. Chicago: SPSS, Inc; 2006. [Google Scholar]
- United States Department of Health and Human Services (USDHHS) Physical activity and health: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services; 1996. [Google Scholar]
- United States Department of Health and Human Services (USDHHS) Healthy People 2010: Understanding and improving health. 2nd. Washington, DC: US Government Printing Office; 2000. [Google Scholar]
- Wells KB, Stewart A, Hays RD, Burnam MA, Rogers W, Daniels M, et al. The functioning and well-being of depressed patients: Results from the Medical Outcomes Study. Journal of the American Medical Association. 1989;262(7):914–919. [PubMed] [Google Scholar]
- Yancey AK, Robinson RG, Ross RK, Washington R, Goodell HR, Goodwin NJ, et al. Discovering the full spectrum of cardiovascular disease: Minority Health Summit 2003: Report of the Advocacy Writing Group. American Heart Association Advocacy Writing Group, Circulation. 2005;111(10):e140–e149. doi: 10.1161/01.CIR.0000157744.30181.FF. [DOI] [PubMed] [Google Scholar]
- Yochim BP, Lequerica A, MacNeill SE, Lichtenberg PA. Cognitive initiation and depression as predictors of future instrumental activities of daily living among older medical rehabilitation patients. Journal of Clinical and Experimental Neuropsychology. 2008;30(2):1–9. doi: 10.1080/13803390701370006. [DOI] [PubMed] [Google Scholar]