Abstract
Objective(s): Coumarin hydroxylase (CYP2A6) is a polymorphic enzyme, and during the last decade has received a lot of attention because it is the principle human nicotine C-oxidase, which activates a number of procarcinogens, and metabolizes drugs.
Materials and Methods: 150 healthy Iranian volunteers, (96 male, 54 female) aged 19 to 63 years old, were given 5 mg coumarin orally after an overnight fast. Urine samples were collected before drug administration and 2, 4 and 8 h after that. The extent and rate of the formation of 7-OH-coumarin (7OHC) was determined by the urinary excretion of the metabolite as measured by the fluorometric method.
Results: The proportion of 7OHC excreted during the first 2 hr compared to the 7OHC excretion at 8 h was the constant and stable individual characteristic for the rate of the formation of 7OHC (2 hr coumarin test). The total amount of 7OHC formed was 32.7±12.4% (mean±SD) of the given dose. On average, 66.0% of 7OHC formed was excreted in 2 hr. No clear-cut polymorphism in the rate of 7OHC formation was found. The total amounts of 7OHC excreted were significantly lower in females. Also, the rate and amount of coumarin metabolism in Iranian population were lower than Turkish and Finnish populations.
Conclusion: Because of the importance of enzyme activity in drug metabolism, caution should be exercised in prescribing compounds metabolized by CYP2A6 enzyme in Iranian population.
Key Words: CYP2A6, Coumarin, Fluorometry, Iran, Polymorphism, 7-hydroxycoumarin
Introduction
There is a pronounced inter-individual and interethnic variability in the levels and activities of many drug-metabolizing enzymes. This could cause inter-individual differences in sensitivities to the effects and toxicity of many clinically used drugs and environmental compounds. In many cases, the basis for this variability is genetic polymorphism in the genes encoding these enzymes (1). Cytochrome P450s (CYPs) are a family of heme-containing enzymes implicated in the metabolism of xenobiotics in the body. Five CYPs (CYP1A2, 2C9, 2C19, 2D6, and 3A4) are responsible for approximately 90% of CYP-mediated drug metabolism in the body, with the remaining CYP enzymes being involved in steroidogenesis and fatty acid oxidation. CYPs catalyze the hydroxylation, epoxidation, and N-, S-, and O-demethylation of drug molecules, allowing for second-phase metabolism and excretion from the body (2, 3). CYP2A6, first identified as the human coumarin 7-hydroxylase, is a polymorphic member of CYP family of enzymes (4, 5). It constitutes 5-10% of the total microsomal CYPs of human liver (6). This enzyme is predominantly expressed in the liver (7) and is responsible for the clearance of many drugs and environmental chemicals. It is also the most important P450 responsible for nicotine C-oxidation and metabolizes cigarette nitrosamines as well (8).
Coumarin is a naturally occurring plant compound with a high safety profile. No adverse effects of coumarin have been reported in susceptible species in response to doses which are more than 100 times the maximum human daily intake (9). Because of its certain biochemical properties, coumarin has been proposed to be used in clinical medicine, as a macrophage activator in the treatment of certain carcinomas (10, 11). It is mainly metabolized by CYP2A6 and is widely used as a probe for the determination of activity of the enzyme (12, 13) and in polymorphism studies (4, 14, 15).
Coumarin 7-hydroxylation is catalyzed by a high-affinity CYP2A6 enzyme in human liver microsomes. CYP2A6 is the only enzyme catalyzing this reaction and consequently the formation of 7-hydroxycoumarin can be used as ‘an in vitro and in vivo probe’ for CYP2A6 (16).
Since CYP2A6 has been identified as the human coumarin 7-hydroxylase and it is responsible for the clearance of many drugs and environmental chemicals, in this study, the activity of coumarin hydroxylase as a marker of the activity of CYP2A6 is investigated in an Iranian population.
Materials and Methods
One hundred and fifty one volunteers (97 male, 54 female), not receiving any drug before or after the test, aged between 19 and 63 (Mean±SD= 23.8±6.6), participated in the study after giving written informed consent. Volunteers were the students or teachers of Mashhad University of Medical Sciences (MUMS), most of them students of the School of Pharmacy (east of Iran). Two of them were permanent smokers and 8 were temporary smokers. The Ethics Committee of Mashhad University of Medical Sciences approved the appropriate standards of the experiments in humans. Also, the volunteers had been given informed consent sheet prior to participating in this study.
Each volunteer took one capsule containing 5 mg coumarin (produced by department of Industrial Pharmacy of the School) with 200 ml water, after an overnight fast. A control urine sample was collected at the same time. Volunteers were allowed to drink 200 ml water 1 hr after and to eat food 3 hr after coumarin administration. Urine was voided 2, 4 and 8 hr after coumarin administration, volumes were recorded, and after centrifugation at 3000 g for 5-10 min a sample (5 ml) was frozen (- 20oC) until analyzed.
Determination of 7-hydroxylation in the urine samples was performed spectrofluorometrically, essentially as described by Rautio et al (17). Briefly, 0.2 ml of urine samples were mixed with 0.3 ml 0.1 M acetate buffer pH 5.0 containing 100 units of β-glucuronidase from bovine liver (obtained from Sigma-Aldrich). The tubes were incubated in a metabolic shaker for 2 hr at 37oC. 0.05 ml of the incubate was withdrawn, mixed with 0.45 ml distilled water and 2 ml dichloromethane. The tubes were closed and shaken for 10 min. at 37oC. One ml of chloroform phase was taken to 2.5 ml of 0.1 M glycine buffer (pH 10.4), vortexed for 10 sec, and alkaline phase was measured immediately by a spectrofluorometer at 365 and 454 nm excitation and emission wavelengths, respectively. Standards and a sample with a known amount of 7OHC were run simultaneously.
For the calculation of the amount and percent of total metabolized coumarin (8-hr test), the following equations was used:
Statistical analysis
Kolmogorov–Smirnov test was used to determine the normal distribution of the data. To determine the significant difference between groups, student t-test was applied and to measure the linear correlation between variables, Pearson’s coefficient of correlation was used.
Results
2-hr coumarin excretion test
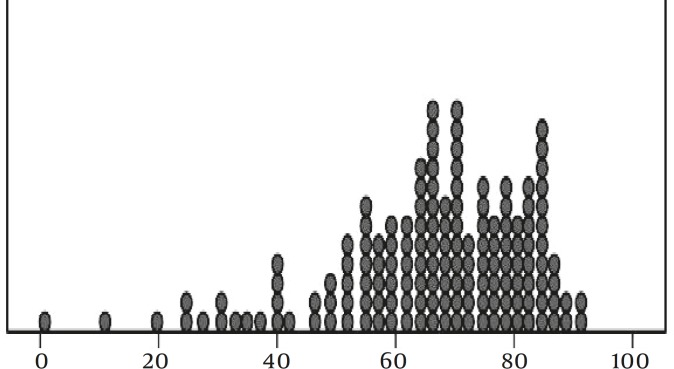
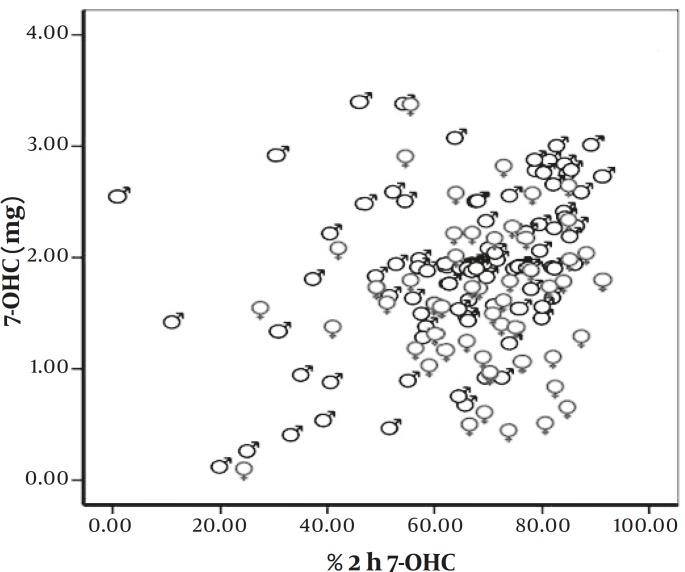
2 hr coumarin excretion test is a basis for the comparison of the rate of CYP2A6 activity between individuals. In 150 healthy volunteers, the total amount of 7OHC recovered from urine in the first 2 hr was in the range of 0.02 and 2.68 mg (mean±SD = 1.22±0.58 mg). Table 1 shows an overall picture of the percentage of 7-OHC excretion in males and females. Figure 1 shows the results based on the percentage of total 7OHC excretion in details. There were not any significant differences between males and females in this respect. The median CYP2A6 activity was 68.62%. Kolmogorov–Smirnov test confirms the normal distribution of the data (P=0.064). The correlation between 2-hr coumarin test and the total amount of excreted 7OHC with sex differentiation is shown in Figure 2.
Figure 1.
7OHC excretion in 2-hr urinary test. 150 Iranian volunteers received a 5 mg capsule of coumarin after an overnight fast and their urinary 7OHC excretion was measured spectrophotometrically.
4-hr coumarin excretion test
Table 2 shows an overall picture of the percentage of 7-OHC excretion in males and females in the first 4 hrs. There was not any significant difference between males and females. The amount of 7OHC excreted in 4 hrs was in the range of 0.05 and 2.98 mg (mean±SD = 1.63±0.64 mg).
8-hr coumarin excretion test
The results are depicted in Table 3. The total amount of 7OHC recovered from urine in 8 hrs was in the range of 0.1 and 3.4 mg (mean±SD = 1.81±0.69 mg). T-test analysis implied a significant difference between males and females in the amount of 7OHC excretion. Kolmogorov–Smirnov test confirms the normal distribution of the data. Generally, 1.89% to 61.33% (mean±SD = 32.74±12.47%) of the given dose of coumarin was metabolized in these volunteers.
Table 2.
Results (percent) of 4-hr 7-OH-coumarin excretion in 150 Iranian volunteers.
| Volunteers | Mean | SD | Minimum | Maximum | No. of volunteers |
|---|---|---|---|---|---|
| Male | 88.93% | 8.38% | 39.78% | 97.53% | 96 |
| Female | 89.35% | 8.59% | 46.32% | 98.49% | 54 |
| Total | 89.08% | 8.43% | 39.78% | 98.49% | 150 |
Table 3.
Results (mg) of the amount of 7-OH-coumarin hydroxylase excretion in 8 hrs
| Volunteers | Mean | SD | Minimum | Maximum | No. of volunteers |
|---|---|---|---|---|---|
| Male | 1.92 mg | 0.68 mg | 0.12 mg | 3.40 mg | 96 |
| Female | 1.62 mg | 0.67 mg | 0.1 mg | 3.38 mg | 54 |
| Total | 1.81 mg | 0.69 mg | 0.1 mg | 3.40 mg | 150 |
Figure 2.
The correlation between 2-hr coumarin test value and the total amount of 7OHC excreted. Male, Female (r = 0.265, P= 0.001, n = 150).
The effect of age and smoking
Volunteers were divided into 2 groups, less than 30 years old and above 30. As it can be seen in Table 4, no significant statistical correlation was found between the age and the amount of 7OHC excreted in 2 hrs. Among 150 volunteers, 2 people were permanent smokers and 8 were recreational smokers. No significant difference between smokers and nonsmokers was found.
Table 4.
Age differentiation in of 2-hr coumarin test in 150 Iranian volunteers
| Volunteers age (year) | Mean | SEM | No. of volunteers |
|---|---|---|---|
| 30 ≤ | 65.99% | 17.08% | 139 |
| < 30 | 66.05% | 10.87% | 11 |
| Total | 65.99% | 16.68% | 150 |
The effects of activity of hepatic enzymes
In this study, the activity of aspartate aminotransferase (SGOT), alanine aminotransferase (ALT) and alkaline phosphatase of volunteers were also determined. A significant difference between the activities of these factors and the amount of the excretion of 7OHC was not found (data not shown). Determination of hepatic enzymes activity was performed to ensure that volunteers are of normal hepatic function, as decreased hepatic function will adversely affect metabolic activity of its enzymes, including CYP2A6.
Discussion
Interindividual and interethnic differences in the activity of drug metabolizing enzymes are of great importance in drug treatment. People with significant difference in drug metabolizing enzyme activity are at higher risk of drug toxicity or poor therapeutic outcome. Cytochrome P450 enzymes are especially important in this respect, as they are responsible for the most reactions of drug metabolism. CYP2A6 (coumarin hydroxylase) is a member of this superfamily expressed in the liver.
In this study, using coumarin 7-hydroxylation, the distribution of CYP2A6 activity in 150 volunteers, aged 19 to 63, in the province of Khorasan Razavi, Iran was investigated. As it was shown that diet has a minor effect on coumarin hydroxylase activity (18), we did not control the diet of volunteers.
CYP2A6 is the only enzyme catalyzing this reaction and consequently the formation of 7-hydroxycoumarin can be used as ‘an in vitro and in vivo probe’ for CYP2A6 (16). The half-life of coumarin in human is 1-1.5 h (19). That is why 2-hr coumarin test is a reliable basis for the interindividual investigation of CYP2A6 activity and was used by numerous investigators in vitro and in vivo. We found a wide variation in 2-hr coumarin test in this study. The results showed a 115-fold interindividual variation of CYP2A6 activity, in the range of 0.79% to 91.27% (Table 1). There was not any significant differences between males and females, although the amount of 7OHC excretion in 8-hr test was significantly higher in males (P<0.01, Table 5). Another study in Iranian population found different alleles distribution compared with other populations (20). A similar study in 100 Turkish volunteers found a higher rate of CYP2A6 activity (74.7% compared with our result 65.99%) (21). A study in Finnish population (110 volunteers) also found a higher mean activity compared with our results, 84.3% compared with 65.99% (17). Chinese investigators found a 300-fold variation in coumarin hydroxylae activity with a bimodal distribution, resulting in the identification of 13.3% of volunteers as poor metabolizers (22). In this study, no breakpoint was found in coumarin hydroxylase activity, but, similar to the Chinese study, a number of volunteers with very low CYP2A6 activity were found.
Table 1.
Results (percent) of 2-hr 7-OH-coumarin excretion in 150 Iranian volunteers.
| Volunteers | Mean | SD | Minimum | Maximum | No. of volunteers |
|---|---|---|---|---|---|
| Male | 65.01% | 17.80% | 0.79% | 91.27% | 96 |
| Female | 67.73% | 14.46% | 24.4% | 91.34% | 54 |
| Total | 65.99% | 16.68% | 0.79% | 91.34% | 150 |
Table 5.
Results (%) of the amount of 7-OH-coumarin hydroxylase excretion in volunteers
| Volunteers | 2 hr | 4 hr | 8 hr | No. of volunteers |
|---|---|---|---|---|
| Male | 65.01±17.80 | 88.93±8.38 | 100 | 96 |
| Female | 67.73±14.46 | 89.35±8.59 | 100 | 54 |
| Total | 65.99±16.68 | 89.08±8.43 | 100 | 150 |
Normal distribution in 2 hr coumarin excretion test implies that a phenotype differentiation could not be found. Similar conclusions were made in Turkish and Finnish studies as well. Researchers made individually defined differentiation points in coumarin hydroxylase activity. Turkish investigators defined slow hydroxylators as volunteers had a 2-hr coumarin test <40%. Finnish investigators defined this border as <60% and Chinese investigators as 47%. 8%, 28% and 10.6% of Iranian volunteers could be defined as poor metabolizers based on Turkish, Finnish and Chinese investigators, respectively. In two studies by other researchers using different markers of CYP2A6 activity in Iranian population and in different parts of the country, different rates of poor metabolizers were identified. In a study conducted on 200 Iranian volunteers in Tehran (centre of Iran), using dextromethorphan as a marker of CYP2D6 activity, poor metabolizers found to be 2.5% (23). In another study, based on genetic determination of CYP2D6 activity in Tabriz (west of Iran), 4% of the population (110 volunteers) were found to be poor metabolizers (24).
The results presented here show the proportion of hydoxylated coumarin in 8-hr test is in the range of 1.89-61.33% (mean±SD = 32.74±12.47%). It was 60% and 65% in Turkish and Finnish populations, respectively.
Conclusion
Collectively, considering the results of 2-hr and 8-hr tests, a comparison between the results presented in this study with Turkish and Finnish population indicates that Iranian population shows a lower rate of coumarin hydroxylase activity. As some important carcinogens such as aflatoxin B1 and nitosamines are metabolized by CYP2A6 (8), the lower activity of CYP2A6 in Iranian population poses them to a lower risk of cancer incidence due to active metabolites of these compounds. This may also be important in the metabolism and half-life of its drug substrates such as halothane and valproic acid. As the most of our volunteers were pharmacy students, some of them from other parts of the country, we can cautiously popularize our results to Iranian population. Although for a more confident conclusion, sampling from other parts of the country is recommended in complementary studies.
Acknowledgment
The authors would like to appreciate the authorities in research council of Mashhad University of Medical Sciences and School of Pharmacy (MUMS) for their financial support. The results presented in this work have been taken from a Pharmacy student’s thesis.
References
- 1.Ingelman-Sundberg M, Oscarson M, McLellan RA. Polymorphic human cytochrome P450 enzymes: an opportunity for individualized drug treatment. Trends Pharmacol Sci. 1999;20:342–349. doi: 10.1016/s0165-6147(99)01363-2. [DOI] [PubMed] [Google Scholar]
- 2.Schneider E, Clark DS. Cytochrome P450 (CYP) enzymes and the development of CYP biosensors. Biosens Bioelectron. 2013;39:1–13. doi: 10.1016/j.bios.2012.05.043. [DOI] [PubMed] [Google Scholar]
- 3.Singh D, Kashyap A, Ram Vinay Pandey RV, Saini KS. Novel advances in cytochrome P450 research. Drug Discov Today. 2011;16:793–799. doi: 10.1016/j.drudis.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Le Gal A, Dreano Y, Lucas D, Berthou F. Diversity of selective environmental substrates for human cytochrome P450 2A6: alkoxyethers, nicotine, coumarin, N-nitrosodiethylamine, and N-nitrosobenzylmethylamine. Toxicol Lett. 2003;144:77–91. doi: 10.1016/s0378-4274(03)00229-7. [DOI] [PubMed] [Google Scholar]
- 5.Miles JS, McLaren AW, Forrester LM, Glancey MJ, Lang MA, Wolf CR. Identification of the human liver cytochrome P-450 responsible for coumarin 7-hydroxylase activity. Biochem J. 1990;267:365–371. doi: 10.1042/bj2670365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamano S, Tatsuno J, Gonzalez FJ. The CYP2A3 gene product catalyzes coumarin 7-hydroxylation in human liver microsomes. Biochemistry. 1990;29:1322–1329. doi: 10.1021/bi00457a031. [DOI] [PubMed] [Google Scholar]
- 7.Koskela S, Hakkola J, Hukkanen J, Pelkonen O, Sorri M, Saranen A, et al. Expression of CYP2A genes in human liver and extrahepatic tissues. Biochem Pharmacol. 1999;57:1407–1413. doi: 10.1016/s0006-2952(99)00015-5. [DOI] [PubMed] [Google Scholar]
- 8.Oscarson M. Genetic polymorphisms in the cytochrome p450 2a6 (cyp2a6) gene: implications for interindividual differences in nicotine metabolism. Drug Metab Dispos. 2001;29:91–95. [PubMed] [Google Scholar]
- 9.Lake BG. Coumarin metabolism, toxicity and carcinogenicity: relevance for human risk assessment. Food Chem Toxicol. 1999;37:423–453. doi: 10.1016/s0278-6915(99)00010-1. [DOI] [PubMed] [Google Scholar]
- 10.Thornes D, Daly L, Lynch G, Browne H, Tanner A, Keane F, et al. Prevention of early recurrence of high risk malignant melanoma by coumarin. Irish Melanoma Group. Eur J Surg Oncol. 1989;15:431–435. [PubMed] [Google Scholar]
- 11.Dexeus FH, Logothetis CJ, Sella A, Fitz K, Amato R, Reuben JM, et al. Phase II study of coumarin and cimetidine in patients with metastatic renal cell carcinoma. J Clin Oncol. 1990;8:325–329. doi: 10.1200/JCO.1990.8.2.325. [DOI] [PubMed] [Google Scholar]
- 12.Gal A, Dre´ano Y, Lucas D, Berthou F. Diversity of selective environmental substrates for human cytochrome P450 2A6: alkoxyethers, nicotine, coumarin, N-nitrosodiethylamine, and N-nitrosobenzylmethylamine. Toxicol Lett . 2003;144:77–91. doi: 10.1016/s0378-4274(03)00229-7. [DOI] [PubMed] [Google Scholar]
- 13.Higashi E, Nakajima M, Katoh M, Tokudome S, Yokoi T. Inhibitory effects of neurotransmitters and steroids on human CYP2A6. Drug Metab Dispos. 2007;35:508–514. doi: 10.1124/dmd.106.014084. [DOI] [PubMed] [Google Scholar]
- 14.Xu C, Goodz S, Sellers EM, Tyndale RF. CYP2A6 genetic variation and potential consequences. Adv Drug Deliv Rev. 2002;54:1245–1256. doi: 10.1016/s0169-409x(02)00065-0. [DOI] [PubMed] [Google Scholar]
- 15.Kim D, Wu ZL, Guengerich FP. Analysis of coumarin 7-hydroxylation activity of cytochrome P450 2A6 using random mutagenesis. J Biol Chem. 2005;280:40319–40327. doi: 10.1074/jbc.M508171200. [DOI] [PubMed] [Google Scholar]
- 16.Pelkonen O, Rautio A, Raunio H, Pasanen M. CYP2A6: a human coumarin 7-hydroxylase. Toxicology . 2000;144:139–147. doi: 10.1016/s0300-483x(99)00200-0. [DOI] [PubMed] [Google Scholar]
- 17.Rautio A, Kraul H, Kojo A, Salmela E, Pelkonen O. Interindividual variability of coumarin 7-hydroxylation in healthy volunteers. Pharmacogenetics. 1992;2:227–233. doi: 10.1097/00008571-199210000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Rauma AL, Rautio A, Pasanen M, Pelkonen O, Torronen R, Mykkanen H. Coumarin 7-hydroxylation in long-term adherents of a strict uncooked vegan diet. Eur J Clin Pharmacol. 1996;50:133–137. doi: 10.1007/s002280050081. [DOI] [PubMed] [Google Scholar]
- 19.Ritschel WA, Brady ME, Tan HS, Hoffmann KA, Yiu IM, Grummich KW. Pharmacokinetics of coumarin and its 7-hydroxy-metabolites upon intravenous and peroral administration of coumarin in man. Eur J Clin Pharmacol. 1977;12:457–461. doi: 10.1007/BF00561066. [DOI] [PubMed] [Google Scholar]
- 20.Emamghoreishi M, Bokaee HR, Keshavarz M, Ghaderi A, Tyndale RF. CYP2A6 Allele Frequencies in an Iranian population. Arch Iran Med. 2008;11:613–617. [PubMed] [Google Scholar]
- 21.Iscan M, Rostami H, Iscan M, Guray T, Pelkonen O, Rautio A. Interindividual variability of coumarin 7-hydroxylation in a Turkish population. Eur J Clin Pharmacol. 1994;47:315–318. doi: 10.1007/BF00191161. [DOI] [PubMed] [Google Scholar]
- 22.Xu P, Huang SL, Xu RH, Han XM, Zhou HH. Phenotypic polymorphism of CYP2A6 activity in a Chinese population. Eur J Clin Pharmacol. 2002;58:333–337. doi: 10.1007/s00228-002-0480-3. [DOI] [PubMed] [Google Scholar]
- 23.Afshar M, Rouini M, Ala S. Dextromethorphan metabolic phenotyping in an Iranian population. Eur J Clin Pharmacol . 2005;60:849–854. doi: 10.1007/s00228-004-0859-4. [DOI] [PubMed] [Google Scholar]
- 24.Kouhi H, Hamzeiy H, Bara J, Asadi M, Omidi Y. Frequency of five important CYP2D6 alleles within an Iranian population (Eastern Azerbaijan) Gnet Test Mol Biomarkers. 2009;13:665–670. doi: 10.1089/gtmb.2009.0009. [DOI] [PubMed] [Google Scholar]