Abstract
Allosteric activation of antithrombin as a rapid inhibitor of factors IXa and Xa requires binding of a high-affinity heparin pentasaccharide. The currently-accepted mechanism involves removal of a constraint on the antithrombin reactive center loop (RCL) so that the proteinase can simultaneously engage both the P1 arginine and an exosite at Y253. Recent results suggest that this mechanism is incorrect in that activation can be achieved without loop expulsion, while the exosite can be engaged in both low and high activity states. We propose a quite different mechanism in which heparin activates antithrombin by mitigating an unfavorable surface interaction, by altering its nature, and by moving the attached proteinase away from the site of the unfavorable interaction through RCL expulsion.
Keywords: antithrombin, heparin activation, proteinase inhibition, factor Xa, factor IXa
1. Introduction
Antithrombin is the principal serpin inhibitor and regulator of the blood coagulation proteinases thrombin, factor Xa and factor IXa [1]. However, in the absence of heparin the second order rate constants for inhibition (k2) of each proteinase are too low to be physiologically effective, with values of 2–8 × 103 M−1 s−1 for factor Xa and thrombin [2] and only 2 × 102 M−1 s−1 for factor IXa [1]. Heparin dramatically enhances rates by two distinct mechanisms. First, a high-affinity pentasaccharide (H5) binds antithrombin and causes allosteric changes that result in 100–300 fold increases in k2 for factors IXa and Xa respectively, but less than one-fold increase for thrombin [2]. Longer heparins can simultaneously bind both antithrombin and each proteinase [3]. This bridging gives produces rate enhancements of ~1000-fold for thrombin, 100-fold for factor IXa and 10-fold for factor Xa.
A fundamental question is how allosteric activation of antitithrombin occurs. With the suggestion from x-ray structures of heparin-free [4,5] and heparin-bound antithrombin [6] that heparin binding might allow greater movement of the reactive center loop (RCL), through expulsion of the latter's constrained N-terminal end, as well as the structure of antithrombin in ternary complex with activating H5 and factor Xa (S195A), in which factor Xa engages both P1 Arg and an exosite containing Y253 [7], it seemed that the answer had been found. Thus, the current, widely accepted mechanism of antithrombin activation is that, with the RCL hinge buried at the top of β-sheet A in the native state, the RCL is constrained such that factor Xa or IXa can bind only to P1 Arg and adjacent residues, but not to the Y253 exosite (Fig 1A). This inability to engage the exosite is proposed to explain the low rate of reaction by these two rather inefficient proteinases. H5 binding expels the buried RCL hinge, giving it sufficient extra unconstrained length to permit either proteinase to simultaneously engage both P1 and the exosite. The extra favorable interaction with the exosite is thus the source of the rate enhancement. In this model a low, but otherwise normal rate of reaction between antithrombin and proteinase, is changed to one in which enhancement arises through additional favorable interactions.
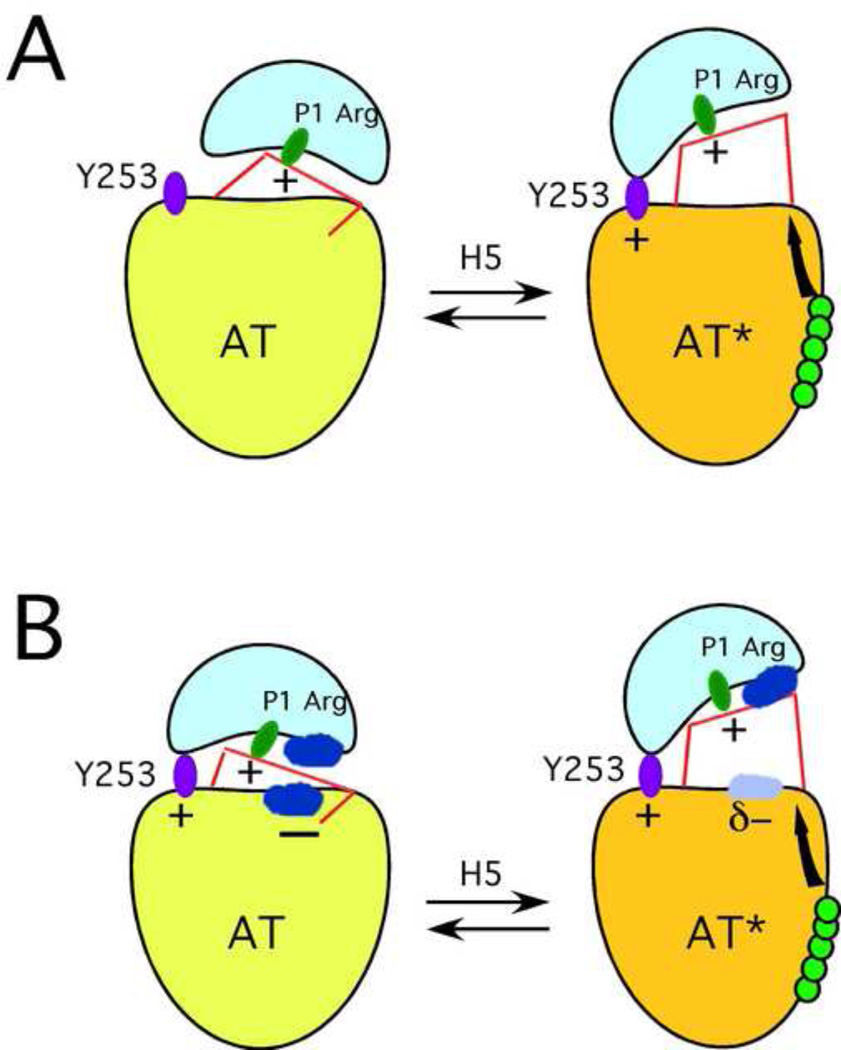
Figure 1. Old and new mechanisms of heparin activation of antithrombin as an inhibitor of factors IXa and Xa.
Panel A, current, old mechanism of activation in which proteinase (blue) only engages P1 Arg (green elipse) in the unactivated state (AT) to give one favorable (+) interaction. H5 (green balls) binding causes conformational changes (black arrow) that result in RCL hinge expulsion, upward and horizontal movement of the proteinase so that it can engage both P1 Arg and Y253, to give two favorable (+,+) interactions. Binding energy differences between serpin and proteinase in the activated vs unactivated states arise from additional engagement of Y253 in the latter. Panel B, new mechanism of activation. In the non-activated state (AT) the proteinase engages both P1 Arg and Y253, but these two favorable interactions (+,+) are partly offset by the repulsion (−) between patches (dark blue) on the proteinase and the serpin. H5 binding causes conformation changes that expel the RCL hinge, but also alter the repulsive region on antithrombin (lighter shade of blue) so that the interaction with the repulsive patch on the proteinase is diminished (interaction reduced from "−" to "δ−"). The energy difference for binding of serpin and proteinase in activated vs unactivated states arises from (i) reduction in the unfavorable interaction from "−" to "δ−", and (ii) movement away of the proteinase from the repulsive patch on antithrombin.
2. Problems with the current mechanism
The most pressing reason to re-evaluate this model was a study just conducted in our laboratories on an antithrombin variant in which residues 131–136 were mutated to α-helix-promoting ones (YRKANK changed to LEEAAE) [8]. This variant had a basal rate of factor Xa inhibition (k2 ~ 3.1 × 105 M−1 s−1) ~ 50-fold higher than wild-type antithrombin, yet properties consistent with the RCL still being inserted into β-sheet A. These included near-normal Trp fluorescence that was enhanced 25% upon binding H5 and only modestly enhanced heparin affinity (2× decrease in Kd). A further 6-fold rate enhancement, together with RCL expulsion, occurred when H5 bound, to give ~3-fold higher k2 than wild-type antithrombin bound to H5. Significantly, an earlier study in which only Y131 was changed to Ala or Leu found a similar effect, with greatly enhanced basal rate of factor Xa inhibition in the absence of heparin (25–29 fold) and a further 8–12 fold enhancement when H5 bound [9]. Since in both cases most of the activation occurred while the RCL hinge was still constrained by β-sheet A, this raised doubts about the role of RCL hinge expulsion as the means of activation through engagement of a new favorable exosite.
Concerning the accessibility and role of the proposed exosite, Y253, in native and heparin-activated states, two quite different published datasets suggest that, while Y253 is indeed engaged by factor Xa and factor IXa, this occurs in both native and heparin-bound states, rather than the exosite being available only in the heparin-activated state. In one study Y253 was mutated and the effects on basal and heparin-activated rates of inhibition of thrombin, factor IXa and factor Xa examined [10]. Replacement with Ala had minimal effect on the basal rate of thrombin inhibition, but reduced the rate of factor IXa inhibition by ~200 fold and of factor Xa by 10-fold. This suggests that Y253 already binds factor IXa and factor Xa in the low activity, loop-inserted state, and must make a major contribution to the interaction. Strikingly the Y253A variant still showed the normal 200-fold rate enhancement upon H5 binding for both factor IXa and factor Xa, even though Y253 was absent. The second type of data to suggest that Y253 is engaged in both heparin-free and heparin-bound states is more circumstantial. In the currently-accepted mechanism, it is suggested that loop expulsion is needed to remove constraints on the RCL so that either factor Xa or factor IXa can simultaneously engage P1 Arg and Y253. This implies that these two groups are too far apart in the native state to permit this. X-ray structures suggest that this is incorrect. The first structures of native antithrombin showed Cα-Cα separation between P1 Arg and Y253 of 16Å [4,5]. A more recent structure had Cα-Cα of a mere 10Å between P1 and Y253 [11]. These structures demonstrate that the RCL is sufficiently flexible in the loop-inserted state for the position of the P1 Cα to move by 6Å, as dictated by circumstances. In three structures of antithrombin in heparin-bound non-covalent Michaelis complex with proteinase the separation between P1 and Y253 Cα is 17–19Å in the two thrombin complexes [3,12] and 19Å in the factor Xa complex [7]. This negates the idea that loop expulsion is needed to provide extra length to the RCL and thereby allow the P1 Arg to move closer to Y253 and so permit factor Xa or factor IXa to engage both residues, since the separation is larger than in the native state. P1 and Y253 are already almost exactly the correct distance apart in the native loop-inserted state. Together with the mutagenesis data indicating that Y253 is important for binding factor IXa or factor Xa in the native state, this supports the idea that, while both Y253 and P1 are bound by the proteinase in both native and activated states, Y253 cannot be the source of the activating rate enhancements for either factor IXa or factor Xa.
3. Hypothesis
We propose a quite different mechanism of allosteric activation of antithrombin against factors IXa and Xa that still recognizes the importance of expulsion of the RCL hinge, but only as the minor of two factors that together produce the overall rate enhancement (Fig 1B). In our new model both P1 Arg and Y253 in native antithombin can be simultaneously engaged by factors IXa and Xa, and both contribute favorably to the binding interaction ("+" in Fig 1B). The overall affinity, and consequently the on-rate that determines k2 for Michaelis complex formation, remains low however, because of additional unfavorable interaction(s) between the proteinase and the surface of the antithrombin ("−" in Fig 1B). If the Y253 exosite were not available in the native state, the binding to P1 Arg, and hence rate of inhibition, would be even lower than is observed. When H5 binds, two distinct changes occur with respect to activation. One is a change on the antithrombin surface, mediated in some way by Y131, that results in decreased unfavorableness of the negative proteinase-antithrombin interaction (from "−" to "δ−" in AT*, Fig 1B). The second is expulsion of the RCL hinge that allows the N-terminal end to move out from the antithrombin surface and hence reduces the repulsive proteinase-antithrombin interaction due to increase in separation. This implies that the repulsive site should lie on the antithrombin surface close to the N-terminal end of the RCL. As with complexes formed with native antithrombin, both P1 Arg and Y253 are engaged in the heparin-activated state. The major energetic, and hence kinetic, difference in formation of the antithrombin-proteinase complex when heparin is bound is that favorable binding contributions from P1 and Y253 are no longer diminished by unfavorable interaction with the antithrombin surface. Since the reduction in the unfavorable contribution arises from (i) change in its nature and (ii) increased separation from the repulsive site, each of these should contribute additively in terms of binding energy and could, in appropriate antithrombin variants, occur as separate events, such as in our recent study [8]. From that study an estimate of the relative changes arising from change in the repulsive epitope and movement of the RCL can be made of 50-fold and 6-fold resepctively, though these exact values need not necessarily apply in wild-type antithrombin, where both processes would occur together when heparin binds and where there are, by definition, no mutations that might alter the nature of the surface alterations.
4. Where is the unfavorable interaction site?
It is not possible at present to unambiguously identify residues on either antithrombin or factors IXa or Xa that interact unfavorably to down-regulate the inhibition rate. However, in studies to identify the putative proteinase exosite on antithrombin, 6 swaps of β-strands or loops from regions on antithrombin close to the RCL were made between α1PI and antithrombin [13], of which only that of s3c, which contains Y253, resulted in loss of activation upon heparin binding. This gave only a two-fold rate enhancement for factor Xa inhibition and a reduction of about 40% for factor IXa inhibition when H5 bound [13]. In addition, the basal rate of factor IXa inhibition was reduced only ~2-fold and that of factor Xa by 20%, despite the absence of Tyr at position 253 and its replacement by Lys. Together, these observations are consistent with the β-strand swap simultaneously removing the unfavorable interaction and the favorable Y253 interaction. Removal of the unfavorable interaction at the same time as loss of the favorable Y253 exosite offset one another to give only a slightly altered basal rate, whereas the removal of the unfavorable interaction eliminated any rate enhancement possible from heparin-induced conformational changes. Of residues close enough to the RCL to be possible sources of difference in behavior in the strand-swap variant, 254QEgK, which were changed to 254RLgM, are likely candidates. Both Q254 and E255 are conserved in the 13 sequenced antithrombins, while K257 is either K or R in 12 [14]. Significantly, these residues lie directly beneath the middle of the RCL and so fulfill the expectation that the repulsive site should be close to where factor Xa would dock with the RCL in the unactivated state.
5. Re-evaluation of other relevant data
Besides our recent data on the 131–136 antithrombin variant, other studies have proved difficult to interpret on the old model, but are more readily explained using our new mechanism. One involved stepwise deletions of antithrombin residues 134–137 [15]. Removal of 134–137 produced a two-fold reduction in basal rate of factor Xa inhibition, but still left a 44-fold enhancement upon heparin binding, despite only 9% fluorescence enhancement. The latter was interpreted as representing a shift in equilibrium between inactive loop-inserted and active loop-expelled states of only ~25% (i.e. giving 1/4th of the expected 40% fluorescence enhancement). However, even there it was recognized that the magnitude of the fluorescence change represented a discrepancy between what might be expected on this simple shift in equilibrium and what was found. This was exacerbated when it was shown that about 1/5th of the normal fluorescence enhancement (i.e. about 8%) arose from W49 and W189 and probably did not reflect loop-expulsion per se [16]. Using our new model, those data can be simply interpreted as the deletions resulting in an inability to expel the RCL hinge upon heparin binding and hence giving only 9% fluorescence enhancement. Without loop-expulsion, the observed enhancement in factor Xa inhibition rate would arise from surface-mediated reduction in the unfavorable antithrombin-factor Xa interaction. Strikingly, the observed 44-fold enhancement is very close to the 50-fold enhancement seen for the 131–136 variant in the absence of heparin [8].
Similar behavior was observed for K133P antithrombin [17]. As with the deletion mutants, heparin binding produced only a small fluorescence enhancement (10%) but still gave ~40-fold enhanced rate of factor Xa inhibition. The new interpretation is that heparin-induced loop expulsion has been blocked by the introduction of Pro and so the only heparin-induced changes that contribute to activation are surface-mediated ones that reduce the repulsive factor Xa-antithrombin interaction.
A third study was on a P14C antithrombin variant in which Cys was derivatized with fluorescein [18]. Here, Cys could only be efficiently derivatized in the heparin-bound state, indicating that RCL hinge expulsion was needed to expose the SH group. It was assumed that the bulky fluorescein would prevent the P14 backbone from reinserting into β-sheet A upon heparin removal. The 200-fold rate enhancement of the derivative with factor Xa was consistent with this and hence with the sufficiency of loop expulsion to give full activation. However, a subsequent x-ray structure of the derivative showed that the RCL hinge was still inserted into β-sheet A and the fluorescein moiety lay on the antithrombin surface close to the RCL N-terminus [19]. The derivative also had normal Trp fluorescence, which was enhanced 38% upon heparin binding, again implying that the RCL hinge is inserted into β-sheet A and is subsequently expelled upon heparin binding (Gettins, P.G.W. and Huntington, J.A., unpublished results). If this interpretation is correct, the highly activated state in the absence of heparin cannot arise from expulsion of the RCL hinge, but instead from some surface change induced by the negatively charged fluorescein.
An antithrombin variant whose behavior could be interpreted by either model is P14E(S380E) [20] (and unpublished data, Roth and Olson). Its fluorescence (40% enhanced intensity compared with wild-type antithrombin, but no change upon binding heparin) indicates that its RCL is fully expelled and its rate of reaction with factor Xa already represents full activation, even in the absence of heparin. Seemingly this correlated with Glu inducing loop expulsion and consequently producing the rate enhancement. However, a P14R variant has Trp fluorescence similar to loop-inserted wild-type antithrombin, gives 20% enhancement upon heparin binding and yet already reacts 50-fold faster with factor Xa (Ryan Roth and Steven Olson, unpublished results). Our model suggests an alternative interpretation in which the charge at P14, whether positive (Arg) or negative (Glu), results in amelioration of the repulsive factor Xa-antithrombin interaction, and that whether the RCL hinge is expelled or not results in only small additional changes.
6. Summary
Published data on the role of Y253 of antithrombin in the inhibition of factors IXa and Xa demonstrate that, although Y253 is a critical exosite, it is used in both native and heparin-activated states and so cannot be the source of heparin-induced rate enhancements. To-be-published data on a helix-D variant of antithrombin further show that RCL hinge expulsion is not required to give very large activation of antithrombin as a factor Xa inhibitor [8]. These observations are inconsistent with the current mechanism of heparin activation, which involves heparin-induced conformational change to expel the RCL hinge and hence permit engagement of the Y253 exosite. Our new model proposes that Y253 is engaged in both native and activated states and so does not contribute to H5-induced rate enhancement. Instead, heparin-induced conformational changes in antithrombin reduce or eliminate a repulsive interaction with factor Xa or IXa that otherwise reduces their rates of reaction with antithrombin, resulting in a 30–50-fold rate enhancement. Additionally, RCL expulsion allows the proteinase to move further away from the repulsive site, giving a further rate enhancement of 6–12 fold. Other studies on antithrombin variants that were difficult to interpret can now be understood using the new mechanism, lending further support to its validity.
Supplementary Material
Acknowledgements
We gratefully acknowledge support through NIH grants HL39888 (STO) and HL49234 (PGWG).
Abbreviations used
- H5
high affinity heparin pentasaccharide
- RCL
reactive center loop
- P1, P2, etc
designation of RCL residues [1] in which the scissile bond is between residues P1 and P1’
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bedsted T, Swanson R, Chuang Y-J, Bock PE, Björk I, Olson ST. Heparin and calcium ions dramatically enhance antithrombin reactvity with factor IXa by generating new interaction exosites. Biochemistry. 2003;42:8143–8152. doi: 10.1021/bi034363y. [DOI] [PubMed] [Google Scholar]
- 2.Olson ST, Björk I, Sheffer R, Craig PA, Shore JD, Choay J. Role of the antithrombin-binding pentasaccharide in heparin acceleration of antithrombin-proteinase reactions. Resolution of the antithrombin conformational change contribution to heparin rate enhancement. J. Biol. Chem. 1992;267:12528–12538. [PubMed] [Google Scholar]
- 3.Dementiev A, Petitou M, Herbert JM, Gettins PGW. The ternary complex of antithrombin-anhydrothrombin-heparin reveals the basis of inhibitor specificity. Nature Structural and Molecular Biology. 2004;11:863–867. doi: 10.1038/nsmb810. [DOI] [PubMed] [Google Scholar]
- 4.Carrell RW, Stein PE, Fermi G, Wardell MR. Biological implications of a 3Å structure of dimeric antithrombin. Structure. 1994;2:257–270. doi: 10.1016/s0969-2126(00)00028-9. [DOI] [PubMed] [Google Scholar]
- 5.Schreuder HA, de Boer B, Dijkema R, Mulders J, Theunissen HJM, Grootenhuis PDJ, Hol WGJ. The intact and cleaved human antithrombin III complex as a model for serpin-proteinase interactions. Nat. Struct. Biol. 1994;1:48–54. doi: 10.1038/nsb0194-48. [DOI] [PubMed] [Google Scholar]
- 6.Jin L, Abrahams JP, Skinner R, Petitou M, Pike RN, Carrell RW. The anticoagulant activation of antithrombin by heparin. Proc. Natl. Acad. Sci. U. S. A. 1997;94:14683–14688. doi: 10.1073/pnas.94.26.14683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson DJD, Li W, Adams TE, Huntington JA. Antithrombin-S195A factor Xa-heparin structure reveals the allosteric mechanism of antithrombin activation. EMBO J. 2006;25:2029–2037. doi: 10.1038/sj.emboj.7601089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dementiev A, Roth R, Isetti G, Olson ST, Gettins PGW. The allosteric mechanism of activation of antithrombin towards inhibition of factor Xa. Two distinct processes contribute synergistically to the rate enhancement by mitagation of a repulsive interaction. submitted. 2009 [Google Scholar]
- 9.Cruz RGCd, Jairajpuri MA, Bock SC. Disruption of a Tight Cluster Surrounding Tyrosine 131 in the Native Conformation of Antithrombin III Activates It for Factor Xa Inhibition. J. Biol. Chem. 2006;281:31668–31676. doi: 10.1074/jbc.M604826200. [DOI] [PubMed] [Google Scholar]
- 10.Izaguirre G, Olson ST. Residues Tyr253 and Glu255 in strand 3 of β-sheet C of antithrombin are key determinants of an exosite made accessible by heparin activation to promote rapid inhibition of factors Xa and IXa. J. Biol. Chem. 2006;281:13424–13432. doi: 10.1074/jbc.M600415200. [DOI] [PubMed] [Google Scholar]
- 11.Johnson DJD, Langdown J, Li W, Luis SA, Baglin TP, Huntington JA. Crystal structure of monomeric native antithrombin reveals a novel reactive center loop conformation. J. Biol. Chem. 2006;281:35478–35486. doi: 10.1074/jbc.M607204200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li W, Johnson DJ, Esmon CT, Huntington JA. Structure of the antithrombin-thrombin-heparin ternary complex reveals the antithrombotic mechanism of heparin. Nature Structural and Molecular Biology. 2004;11:857–862. doi: 10.1038/nsmb811. [DOI] [PubMed] [Google Scholar]
- 13.Izaguirre G, Zhang W, Swanson R, Bedsted T, Olson ST. Localization of an antithrombin exosite that promotes rapid inhibition of factors Xa and IXa on heparin activation of the serpin. J. Biol. Chem. 2003;279:51433–51440. doi: 10.1074/jbc.M309266200. [DOI] [PubMed] [Google Scholar]
- 14.Backovic M, Gettins PGW. Insights into the function of antithrombin from an expanded database of sequences. Journal of Proteome Research. 2002;1:367–373. doi: 10.1021/pr025515z. [DOI] [PubMed] [Google Scholar]
- 15.Meagher JL, Olson ST, Gettins PGW. Critical role of the linker region between helix D and strand 2A in heparin activation of antithrombin. J. Biol. Chem. 2000;275:2698–2704. doi: 10.1074/jbc.275.4.2698. [DOI] [PubMed] [Google Scholar]
- 16.Meagher JL, Beechem JM, Olson ST, Gettins PGW. Deconvolution of the fluorescence emission spectrum of human antithrombin and identification of the tryptophan residues that are responsive to heparin binding. J. Biol. Chem. 1998;273:23283–23289. doi: 10.1074/jbc.273.36.23283. [DOI] [PubMed] [Google Scholar]
- 17.Belzar KJ, Zhou A, Carrell RW, Gettins PGW, Huntington JA. Helix D elongation and allosteric activation of antithrombin. J. Biol. Chem. 2002;277:8551–8558. doi: 10.1074/jbc.M110807200. [DOI] [PubMed] [Google Scholar]
- 18.Huntington JA, Gettins PGW. Conformational conversion of antithrombin to a fully activated substrate of factor Xa without need for heparin. Biochemistry. 1998;37:3272–3277. doi: 10.1021/bi972182o. [DOI] [PubMed] [Google Scholar]
- 19.Huntington JA, McCoy A, Belzar KJ, Pei XY, Gettins PGW, Carrell RW. A 2.85-Å structure of antithrombin variant S380C-fluorescein reveals the trigger for allosteric activation. J. Biol. Chem. 2000;275:15377–15383. doi: 10.1074/jbc.275.20.15377. [DOI] [PubMed] [Google Scholar]
- 20.Futamura A, Gettins PGW. Serine 380 (P14)→glutamate mutation activates antithrombin as an inhibitor of factor Xa. J. Biol. Chem. 2000;275:4092–4098. doi: 10.1074/jbc.275.6.4092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.