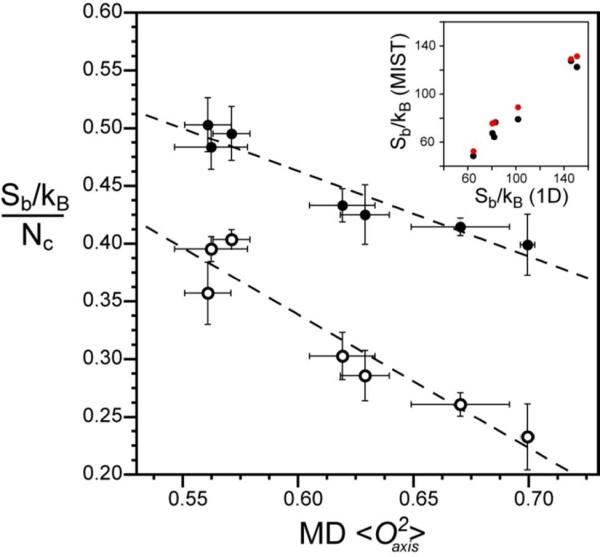
Figure 4.
The dynamic proxy of methyl groups is an excellent reporter of both methyl and total side-chain rotameric entropy. (o) The normalized methyl rotameric entropy for each protein is calculated as the summation of Sb for individual methyl-bearing amino acids divided by the number of associated rotamer angles (Nχ). ( ) The total rotameric entropy for each protein is calculated as the summation of Sb for all residues and is normalized by the respective total number of rotamer angles (Nχ). The average methyl O2axis parameter for all methyl-bearing residues including Ala is that obtained from MD simulations. A very high linear correlation is observed for both methyl-side chain rotamer entropy [slope = −1.16 ± 0.17, R2 = 0.90] and total rotamer entropy [slope = −0.74 ± 0.10, R2 = 0.91 ]. The inset shows the correlation of the uncorrected entropy with the entropy corrected for correlated motions using (
) The total rotameric entropy for each protein is calculated as the summation of Sb for all residues and is normalized by the respective total number of rotamer angles (Nχ). The average methyl O2axis parameter for all methyl-bearing residues including Ala is that obtained from MD simulations. A very high linear correlation is observed for both methyl-side chain rotamer entropy [slope = −1.16 ± 0.17, R2 = 0.90] and total rotamer entropy [slope = −0.74 ± 0.10, R2 = 0.91 ]. The inset shows the correlation of the uncorrected entropy with the entropy corrected for correlated motions using ( ) second–order and (
) second–order and ( ) third-order MIST estimates of inter-residue correlations. Both correlations are highly linear with a slope of −0.89 (R2 = 0.99) and slope = −0.83 (R2 = 0.96) for second-order and third-order MIST calculations, respectively.
) third-order MIST estimates of inter-residue correlations. Both correlations are highly linear with a slope of −0.89 (R2 = 0.99) and slope = −0.83 (R2 = 0.96) for second-order and third-order MIST calculations, respectively.