Figure 4.
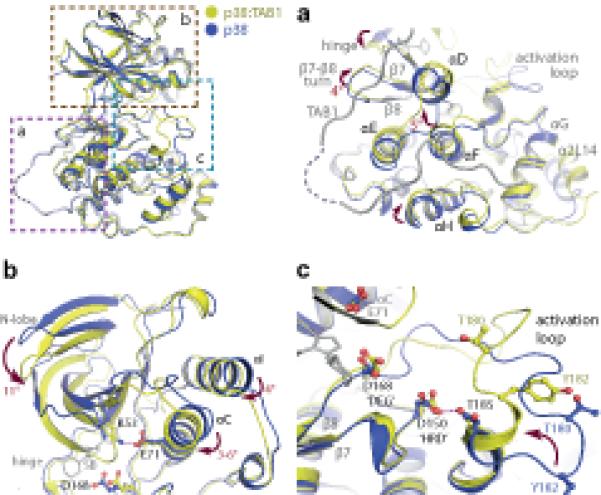
Overview of the key rearrangements within p38α on complex formation with TAB1(384-412). All comparisons are with PDB:1p38 (blue). (a) The accommodation of TAB1 peptide causes displacements in the C-terminal lobe of p38α. Amongst these is an approximate 5° swing of αF. This helix is thought to act as a key register aligning kinase regulatory and catalytic spines (see text). (b) Associated alterations in the N-terminal lobe. A downward swing approximates the 2 lobes of the kinase and is accompanied by a movement of αC towards the ATP binding pocket allowing a salt bridge to form between Lys53 and Glu71. (c) Reordering of the activation loop. The short α-helix at the C-terminus of the activation segment becomes extended. This swings Thr180 towards the key Asp residues (168 and 150) coordinating ATP binding. This loop is stabilised by an interaction between Asp150 and Thr185 that may mimic the function of the HRD motif in coordinating substrates.
