Abstract
Background
The objective of this study was to define clinicopathologic characteristics in concurrent primary thyroid cancer detected by initial 18fluorine-fluorodeoxyglucose positron emission tomography-computed tomography (18F-FDG PET-CT) scanning in patients with underlying malignancy.
Patients and Methods
Among 155 patients with known underlying malignancy and with focal FDG uptake in the thyroid, 25 patients (22 females; mean age±SD 54.4±11.2 years; age range 27–70 years) who were confirmed as having papillary thyroid cancer (PTC; synchronous thyroid cancer) by cytological examination were included. Another 25 patients (24 females; mean age±SD, 48.8±12.7 years) with focal uptake in preoperative 18F-FDG PET-CT due to PTC and no history of other malignancy (primary thyroid cancer) were also included. Immunohistochemical studies were performed for glucose transporter-1 (GLUT-1) and vascular endothelial growth factor (VEGF).
Results
GLUT-1 expression was significantly lower in synchronous thyroid cancer (7 of 25 patients, 28%) compared with primary thyroid carcinoma (15 of 25 patients, 60%; p=0.045). However, age and tumor size of synchronous thyroid cancer were not significantly different from the patients with primary thyroid carcinomas. There was no significant difference in VEGF expression, maximal standardized uptake values, extrathyroidal extension, lymph node metastasis, advanced stage, and multifocality between both thyroid cancer groups.
Conclusion
Clinicopathologic characteristics of synchronous thyroid cancer in patients with underlying malignancy were not different from those of patients with primary thyroid cancers except for GLUT-1 expression.
Introduction
Owing to technical advances such as 18fluorine-fluorodeoxyglucose positron emission tomography-computed tomography (18F-FDG PET-CT) in the early diagnosis and treatment of cancer and an increase in elderly patients, diagnosis of multiple primary cancers (MPC) in one patient has been commonly seen in recent decades. In patients with underlying malignancy, the incidence of synchronous or metachronous multiple primary cancers has increased (1,2). Interestingly, clinically occult second malignancies in the thyroid are increasingly common (3–8). Although the pathogenetic mechanisms underlying MPC are yet to be elucidated, various causes such as heredity and genetic alterations, pollutants, environment, constitution, immunology, carcinogens, radiotherapy, and chemical treatments have been suggested (9).
In recent years, the detection of incidental thyroid nodules by 18F-FDG PET-CT scanning has been commonly reported, and a concern regarding the clinicopathologic characteristics of these incidental thyroid nodules has also arisen. Focal 18F-FDG uptake in the thyroid on 18F-FDG PET-CT is clinically significant because it is related to a high risk of malignancy ranging between 25% and 50% (3–8). FDG is well concentrated in cells with high glycolytic rates, such as poorly differentiated, proliferating thyroid cancer cells. These lesions potentially possess more aggressive behaviors and an unfavorable prognosis (10–12). It has been suggested that this may be due to overexpression of high-affinity glucose transporter-1 (GLUT-1) in the membranes of thyroid tumor cells associated with a more malignant biological behavior (13).
The risk of thyroid cancer increases after the occurrence of other types of primary cancers, and, vice versa, the risk of other types of cancers in other parts of the body increases in patients with differentiated thyroid cancer (DTC). In particular, the chances of developing a second malignancy after primary thyroid or breast cancer may be up to 30% higher compared with the general population (14,15). A recent study showed that unfavorable factors such as lymph node metastasis and invasion into the surrounding soft tissue were significantly more common in patients with multiple tumors than with DTC alone (16).
However, to date, no definitive studies have identified the clinicopathologic characteristics in synchronous primary thyroid cancers detected by 18F-FDG PET-CT in patients with underlying malignancy. The objective of this study was to define the clinicopathologic characteristics in concurrent primary thyroid cancers detected by initial 18F-FDG PET-CT scanning in patients with nonthyroidal primary malignancies.
Patients and Methods
Patients
A total of 155 patients who showed focal thyroid uptake of FDG on whole body 18F-FDG PET-CT while they were evaluated for initial staging of another malignancy (between January 2006 and December 2009 in Pusan National University Hospital) were included in the current study. Among these 155 patients, immunohistochemical studies were performed on 25 patients who were diagnosed with papillary thyroid cancer (PTC; synchronous thyroid cancer) by a cytological examination. All 25 patients underwent total thyroidectomy. Other thyroid incidentalomas included 101 cases of adenomatous goiters, 9 cases of chronic thyroiditis, 13 cases of nodular hyperplasia, 3 cases of Hashimoto's thyroiditis, and 4 cases of cystic changes. The primary underlying cancers were breast cancer (10 patients; 40%), uterine cervical cancer (6 patients; 24%), colorectal cancer (5 patients; 20%), ovarian cancer (1 patient; 4%), diffuse large B-cell lymphoma (1 patient; 4%), gastric cancer (1 patient; 4%), and thymic cancer (1 patient; 4%). Another 40 patients who were diagnosed with primary PTC by a cytological examination were independently enrolled in this study after obtaining consent. They underwent 18F-FDG PET-CT scanning before surgery between March 2009 and December 2009. They had no previous history of malignancy and irradiation. Among them, 15 patients were excluded from the study due to the absence of visible FDG uptake. Thus, 25 patients with primary thyroid cancer were included in this analysis. The clinicopathologic and immunohistochemical features of each group were retrospectively analyzed. This study was approved by the Research Ethics Committee of our institute. Written consent was obtained from all the patients.
18F-FDG PET-CT
18F-FDG PET-CT images were obtained with a PET-CT scanner (Gemini; Philips, Milpitas, CA), consisting of a germanium oxyorthosilicate full-ring PET scanner and a dual-slice helical CT scanner. The 18F-FDG PET-CT images were reviewed by experienced nuclear physicians being blinded to the results of other imaging modalities and pathology results. Treatment regimens, other medical imaging results, and fine-needle aspiration biopsy results were reviewed and analyzed. The PET-CT images were independently reviewed by two experienced nuclear physicians, and any disagreement was resolved by consensus. To calculate maximal standardized uptake values (SUVmax), manually defined circular regions of interest (ROI) were drawn on the attenuation corrected emission images throughout axial planes in which a suspicious lesion could be delineated.
Immunohistochemistry
Surgical specimens were fixed in 10% buffered formalin (pH 7.0) as soon as they were obtained. All sections containing both tumor and surrounding thyroid tissues were embedded in paraffin, and serial sections (4 μm) were cut from a selected, paraffin-embedded tissue block. For histopathologic diagnosis, one of these sections was stained with hematoxylin and eosin (H&E). The other sections were used for immunohistochemistry. Four μm sections from paraffin blocks were transferred to poly-l-lysine coated glass slides and air-dried overnight at 37°C. The glass slides were deparaffinized in xylene (three changes), rehydrated in a graded series of decreasing ethanol concentrations, and then rinsed in Tris-buffered saline (50 mM Tris/HCl pH 7.4 containing 100 mM sodium chloride). Endogenous peroxidase activity was inactivated with 5% hydrogen peroxide in methanol for 15 minutes at 37°C.
After an appropriate antigen retrieval procedure, primary antibodies were applied to the sections. An antibody against glucose transporter-1 (GLUT-1, rabbit polyclonal antibody; DAKO, Glostrup, Denmark) was incubated with the tissue sections at room temperature for one hour. An antibody against VEGF (rabbit polyclonal antibody; Santa Cruz Biotechnology, Santa Cruz, CA) was incubated with the sections overnight at 4°C. Immunohistochemical examination was performed using a DAKO Envision plus for GLUT-1 and VEGF. The reaction products were visualized by exposing sections to 3,3′-diaminobenzidine. Nuclei were lightly counterstained for about 20 seconds with Mayer's hematoxylin. Sections were then mounted in diluted malinol after the application of Universal Mount (DAKO, Carpinteria, CA). In each staining batch, appropriate positive control specimens were used.
Scoring of immunohistochemistry
The staining was independently scored by two different experienced pathologists who were blinded to the clinicopathological data and clinical outcomes of the patients. The scores of two pathologists were compared, and any discrepant scores were reevaluated by re-examining the stainings by both pathologists to achieve a consensus score. With light microscopy, all the tissue sections were scored semi-quantitatively considering the proportion of the cells showing immunoreactivity among whole tumor cells. In each analysis, the percentages of strongly immunoreactive tumor cells among the total tumor cells was visually analyzed in several lower fields (original magnification ×10) covering the entire specimen, and the average percentage was calculated and scored on four-point scales (−, <10%; +, 10–25%; ++, 26–50%; +++, >50%).
Statistical analyses
Statistical analyses of this study were performed using the SPSS (SPSS v15.0 for Windows, Inc., Chicago, IL) software package. Numeric data were expressed as the mean±standard deviation (SD). Categorical data were presented as frequency and percentage. Student's t-test for the differences of nominal variables between two thyroid cancer groups was performed. Fisher's exact test was used to compare expression of GLUT-1 and VEGF between the two thyroid cancer groups and to analyze correlations of clinicopatholgic outcomes according to the results of the immunohistochemical staining in each group. Statistical significance was set at p<0.05.
Results
Patient characteristics
Twenty-five patients (mean age±SD, 54.4±11.2 years; 22 females) with synchronous thyroid cancer detected during the evaluation of the initial staging of another malignancy, and 25 patients (24 females; mean age±SD, 48.80±12.76 years) with a primary thyroid cancer were included in this analysis. At the time of initial presentation, TNM stages of the synchronous thyroid cancers were T1 (n=6), T3 (n=17), T4a (n=2), N0 (n=12), N1a (n=8), N1b (n=5), and M0 (n=25). The TNM stages of the primary thyroid cancers were T1 (n=6), T2 (n=1), T3 (n=18), N0 (n=7), N1a (n=11), N1b (n=7), and M0 (n=25). The characteristics of the patients are summarized in Tables 1 and 2.
Table 1.
Characteristics of Patients with Synchronous Thyroid Cancers
| Patient no. | Sex | Age (yr) | Size (cm) | T | N | M | Stage | Primary cancer |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 45 | 0.9 | 1 | 1a | 0 | III | Cervix cancer |
| 2 | M | 46 | 0.5 | 3 | 1a | 0 | III | Rectal cancer |
| 3 | F | 52 | 1.0 | 3 | 1a | 0 | III | Breast cancer |
| 4 | F | 53 | 1.2 | 1 | 0 | 0 | I | Rectal cancer |
| 5 | F | 53 | 0.9 | 3 | 1a | 0 | III | DLBCL |
| 6 | F | 60 | 1.2 | 3 | 1b | 0 | IVa | Ovarian cancer |
| 7 | F | 66 | 1.2 | 3 | 0 | 0 | III | Cervix cancer |
| 8 | F | 67 | 1.2 | 3 | 1b | 0 | IVa | Colon cancer |
| 9 | F | 68 | 1.1 | 3 | 1b | 0 | IVa | Breast cancer |
| 10 | F | 80 | 1.3 | 3 | 0 | 0 | III | Cervix cancer |
| 11 | F | 40 | 1.8 | 1 | 1a | 0 | I | Breast cancer |
| 12 | F | 56 | 1.8 | 3 | 1b | 0 | IVa | Thymic cancer |
| 13 | F | 57 | 0.8 | 1 | 0 | 0 | I | Breast cancer |
| 14 | F | 50 | 1.2 | 1 | 0 | 0 | I | Breast cancer |
| 15 | F | 45 | 1.3 | 1 | 0 | 0 | I | Breast cancer |
| 16 | M | 70 | 0.6 | 3 | 0 | 0 | III | Colon cancer |
| 17 | F | 53 | 1.1 | 3 | 1a | 0 | III | Cervix cancer |
| 18 | F | 58 | 1.5 | 3 | 0 | 0 | III | Cervix cancer |
| 19 | F | 26 | 1.0 | 3 | 0 | 0 | I | Cervix cancer |
| 20 | F | 46 | 1.2 | 3 | 0 | 0 | III | Breast cancer |
| 21 | F | 50 | 1.4 | 4a | 1b | 0 | IVa | Gastric cancer |
| 22 | F | 58 | 1.8 | 3 | 1a | 0 | III | Breast cancer |
| 23 | F | 52 | 0.5 | 3 | 0 | 0 | III | Breast cancer |
| 24 | F | 45 | 3.4 | 3 | 1a | 0 | III | Breast cancer |
| 25 | M | 64 | 0.8 | 4a | 0 | 0 | IVa | Colon cancer |
Stage, AJCC stage; DLBCL, diffuse large B-cell lymphoma; cervix cancer, uterine cervical cancer.
Table 2.
Characteristics of Patients with Primary Thyroid Cancer Without Underlying Malignancy
| Patient no. | Sex | Age (yr) | Size (cm) | T | N | M | Stage |
|---|---|---|---|---|---|---|---|
| 1 | F | 54 | 0.8 | 1 | 0 | 0 | I |
| 2 | F | 32 | 1.7 | 1 | 1a | 0 | I |
| 3 | F | 53 | 1.4 | 3 | 1b | 0 | IVa |
| 4 | F | 21 | 1.7 | 3 | 1a | 0 | I |
| 5 | F | 51 | 1.1 | 3 | 1a | 0 | III |
| 6 | F | 48 | 1.6 | 3 | 1a | 0 | III |
| 7 | F | 41 | 2.1 | 3 | 1b | 0 | I |
| 8 | F | 71 | 0.9 | 3 | 1b | 0 | IVa |
| 9 | F | 61 | 1.8 | 3 | 1b | 0 | IVa |
| 10 | F | 55 | 0.8 | 3 | 1a | 0 | III |
| 11 | F | 53 | 1.4 | 1 | 1b | 0 | IVa |
| 12 | F | 76 | 1.2 | 3 | 0 | 0 | III |
| 13 | F | 56 | 2.2 | 3 | 1a | 0 | III |
| 14 | F | 51 | 1.3 | 3 | 0 | 0 | III |
| 15 | F | 37 | 2.4 | 2 | 0 | 0 | I |
| 16 | F | 53 | 1.6 | 1 | 1b | 0 | IVa |
| 17 | M | 30 | 3.6 | 3 | 1a | 0 | I |
| 18 | F | 25 | 4.7 | 3 | 1a | 0 | I |
| 19 | F | 50 | 0.9 | 3 | 0 | 0 | III |
| 20 | F | 46 | 1.1 | 3 | 1a | 0 | III |
| 21 | F | 61 | 1.1 | 3 | 1a | 0 | III |
| 22 | F | 49 | 1.3 | 3 | 0 | 0 | III |
| 23 | F | 51 | 0.8 | 3 | 0 | 0 | III |
| 24 | F | 46 | 1.1 | 1 | 1a | 0 | III |
| 25 | F | 49 | 0.5 | 1 | 1b | 0 | IVa |
Immunohistochemical and clinicopathologic characteristics
Figure 1 illustrates representative cases of immunohistochemical staining results of the current study. Table 3 summarizes the clinicopathologic characteristics and immunohistochemical results. The expression rate of GLUT-1 was significantly higher in primary thyroid cancers (p=0.045). However, there was no significant difference in the expression of VEGF, age, size, SUVmax, and clinical outcomes between both thyroid cancer groups.
FIG. 1.
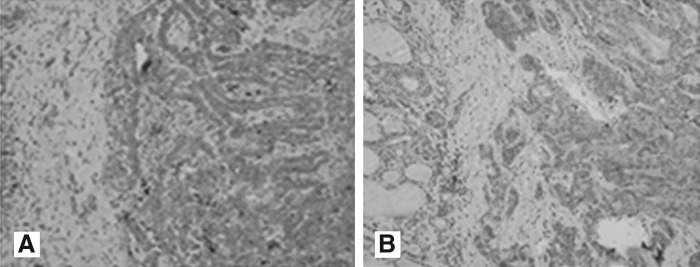
Immunohistochemical positive staining representing the expression profiles for GLUT-1 and VEGF. (A) GLUT-1; (B) VEGF (streptavidin-biotin, ×200). GLUT-1, glucose transporter 1; VEGF, vascular endothelial growth factor.
Table 3.
Comparison Between Synchronous Thyroid Cancer Patients and Primary Thyroid Cancer Patients Without Underlying Malignancy
| Variables | Synchronous thyroid cancer (n=25) | Primary thyroid cancer (n=25) | p-Value |
|---|---|---|---|
| Age (years) | 54.40±11.20 | 48.80±12.76 | 0.106a |
| Size (cm) | 1.23±0.58 | 1.56±0.92 | 0.129a |
| SUVmax | 4.92±3.19 | 6.86±7.02 | 0.214a |
| GLUT-1 | |||
| Positive | 7 (28.0) | 15 (60.0) | 0.045b |
| Negative | 18 (72.0) | 10 (40.0) | |
| VEGF | |||
| Positive | 22 (88.0) | 19 (76.0) | 0.463b |
| Negative | 3 (12.0) | 6 (24.0) | |
| Extrathyroidal extension | 17 (68.0) | 18 (72.0) | 0.652b |
| Lymph node metastasis | 13 (52.0) | 18 (72.0) | 0.145b |
| Advanced stage | 19 (76.0) | 18 (72.0) | 0.747b |
| Multifocality | 6 (24.0) | 5 (20.0) | 0.733b |
Numeric data are expressed as the mean±SD. Categorical data are presented as frequency (percentage).
Student's t-test.
Fisher's exact test.
SD, standard deviation; SUV, standardized uptake value; GLUT-1, glucose transporter 1; VEGF, vascular endothelial growth factor. Advanced stage: TNM stage III+stage IV.
In terms of correlations between clinicopathologic characteristics according to the results of the immunohistochemical staining in synchronous thyroid cancers, VEGF expression was significantly associated with extrathyroidal extension (Table 4; p=0.001). However, biological variables such as GLUT-1 and VEGF did not show any significant correlation with extrathyroidal extension, lymph node metastasis, and advanced tumor stage in patients with primary thyroid cancers (Table 5).
Table 4.
Correlations of Clinicopathologic Characteristics According to Immunohistochemical Results in Synchronous Thyroid Cancer
| |
|
Extrathyroidal extension |
LN metastasis |
Advanced stage |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Biological variables | No | Yes | p-Value | No | Yes | p-Value | I+II | III+IV | p-Value | |
| GLUT-1 | − | 6 | 12 | 0.080 | 10 | 8 | 0.225 | 6 | 12 | 0.080 |
| + | 0 | 7 | 2 | 5 | 0 | 7 | ||||
| VEGF | − | 3 | 0 | 0.001 | 1 | 2 | 0.588 | 2 | 1 | 0.065 |
| + | 0 | 19 | 11 | 11 | 4 | 18 | ||||
p-Values were determined using Fisher's exact test.
LN, lymph node.
Table 5.
Correlations of Clinicopathologic Characteristics According to Immunohistochemical Results in Primary Thyroid Cancer Without Underlying Malignancy
| |
|
Extrathyroidal extension |
LN metastasis |
Advanced stage |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Biological variables | No | Yes | p-Value | No | Yes | p-Value | I+II | III+IV | p-Value | |
| GLUT-1 | − | 3 | 7 | 0.856 | 4 | 6 | 0.275 | 3 | 7 | 0.856 |
| + | 4 | 11 | 3 | 12 | 4 | 11 | ||||
| VEGF | − | 3 | 3 | 0.298 | 1 | 5 | 0.637 | 2 | 4 | 0.742 |
| + | 4 | 15 | 6 | 13 | 5 | 14 | ||||
p-Values were determined using Fisher's exact test.
Discussion
In this study, the cancer risk of incidentally found focal thyroid uptake during the initial 18F-FDG PET-CT scanning in patients with another underlying malignancy was 16% (25/155). The expression of GLUT-1 in primary thyroid cancers was significantly higher compared with that of synchronous thyroid cancers. However, there was no significant difference in age, tumor size, SUVmax, expression of VEGF, and clinicopathologic characteristics between the two thyroid cancer groups.
The clinical course of DTC is typically so indolent that a long life expectancy is common, even if the disease has already progressed to an advanced stage. However, patients with DTC may have an increased likelihood of developing other primary malignancies. Conversely, in patients with another underlying malignancy, the incidence of synchronous or metachronous multiple primary cancers, including thyroid cancer, is increased (1,2). The risk of malignancy in lesions with incidental thyroid uptake during a 18F-FDG PET-CT scanning is reported to be between 26% and 50% (3–8). In the current study, the thyroid cancer risk was 16% in patients with another underlying malignancy when focal thyroid uptake was incidentally found during the initial 18F-FDG PET-CT scan, which was lower than that in previous studies (5–8). FDG uptake was detected more frequently in patients with poorly differentiated thyroid carcinomas, in whom no detectable iodine uptake could be demonstrated. FDG PET-CT avidity is well known to be a poor prognostic factor in PTC (11–13).
Recently, Omür et al. demonstrated that lymph node metastasis and invasion into the soft tissue was significantly more common in patients with multiple tumors than DTC alone (16). Conversely, patients with a past medical history of cancer tend to undergo regular medical check-ups, including 18F-FDG PET-CT, leading to earlier diagnoses of other primary malignancies such as DTC at early stages (7,8). For these reasons, concurrent DTC with other primary malignancies does not always imply a poor prognosis. However, to date, no study is available on the clinicopathologic characteristics of synchronous primary thyroid cancers in patients with underlying malignancy. In our study, there was no significant difference in the expression of VEGF, age, size, SUVmax, and clinicopathologic characteristics between synchronous thyroid cancers and primary thyroid cancers except for GLUT-1 expression, which was higher in patients with primary thyroid carcinomas.
GLUT-1 has been reported to be associated with 18F-FDG uptake in patients with thyroid cancer. An overexpression of high-affinity GLUT-1 in the membranes of thyroid tumors cells is associated with a more malignant biological behavior (13). Kaida et al. recently showed that there were significant correlations between the expressions of GLUT-3, GLUT-4, and SUVmax in thyroid cancer (17). As no or weak GLUT-1 expression was observed in the membrane of DTC, the expression of GLUT-1 was not correlated with SUVmax (17). Park et al. demonstrated that GLUT-1, p63, and p53 were not expressed in well-differentiated thyroid carcinomas, whereas GLUT-1, p63, and p53 were usually expressed in the late stage during the course of thyroid tumor progression (18). Recently, Grabellus et al. showed that the loss of differentiation in thyroid neoplasms is accompanied by the upregulation of GLUT-1, allowing cancer cells to meet their excessive glucose demand to proliferate (19). In our study, the expression of GLUT-1 was significantly higher in primary thyroid cancers compared with that of synchronous thyroid cancers. Thus, it can be assumed that the loss of differentiation was more common in primary thyroid cancer cells compared with synchronous thyroid cancer cells. However, there are no previous studies documenting the comparison of GLUT-1 expression in these thyroid cancers. In addition, because of limited sample size, the high expression of GLUT-1 in primary thyroid cancer observed in our study is considered to be an incidental finding.
Baek et al. showed that the expression of VEGF was significantly associated with nodal metastasis and extracapsular spread in PTC (20). In the current study, VEGF expression was significantly associated with extrathyroidal extension in synchronous thyroid cancer. However, there was no significant correlation with VEGF in primary thyroid cancers. In addition, there was no significant correlation between VEGF expression and lymph node metastasis, as well as advanced tumor stage, in both thyroid cancer groups.
Our study has some potential limitations, which are largely due to its small sample size and the use of immunohistochemistry techniques. In addition, we could not define the relationship between VEGF expression and extrathyroidal extension. These findings warrant further studies.
In conclusion, our results suggest that there is no difference in clinicopathological characteristics and the studied biological markers between synchronous thyroid cancers and primary thyroid cancers. To our knowledge, this is the first study describing the clinicopathologic features of synchronous thyroid cancers in patients with another primary malignancy. However, further studies with a large sample size are needed to elucidate the clinicopathologic features and long-term prognosis of synchronous thyroid cancers with other primary malignancies.
Acknowledgments
This study was supported by a Biomedical Research Institute Grant (No. 2010-23) from Pusan National University Hospital and a grant from the Korean Heath Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (A070001).
Author Disclosure Statement
None of the authors of this study have any conflicts of interest.
References
- 1.Koide N. Yazawa K. Koike S. Adachi W. Amano J. Oesophageal cancer associated with other primary cancers. A study of 31 patients. J Gastroenterol Hepatol. 1997;12:690–694. doi: 10.1111/j.1440-1746.1997.tb00536.x. [DOI] [PubMed] [Google Scholar]
- 2.Naomoto Y. Haisa M. Yamatsuji T. Shirakawa Y. Muramatsu T. Isozaki H. Kamikawa Y. Tanaka N. Multiple primary cancers of the esophagus and thyroid gland. Jpn J Clin Oncol. 1999;29:349–352. doi: 10.1093/jjco/29.7.349. [DOI] [PubMed] [Google Scholar]
- 3.King DL. Stack BC., Jr Spring PM. Walker R. Bodenner D. Incidence of thyroid carcinoma in fluorodeoxyglucose positron emission tomography-positive thyroid incidentalomas. Otolaryngol Head Neck Surg. 2007;137:400–404. doi: 10.1016/j.otohns.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 4.Boeckmann J. Bartel T. Siegel E. Bodenner D. Stack BC., Jr Can the pathology of a thyroid nodule be determined by positron emission tomography uptake? Otolaryngol Head Neck Surg. 2012;146:906–912. doi: 10.1177/0194599811435770. [DOI] [PubMed] [Google Scholar]
- 5.Choi JY. Lee KS. Kim HJ. Shim YM. Kwon OJ. Park K. Baek CH. Chung JH. Lee KH. Kim BT. Focal thyroid lesions incidentally identified by integrated 18F-FDG PET/CT: clinical significance and improved characterization. J Nucl Med. 2006;47:609–615. [PubMed] [Google Scholar]
- 6.Soelberg KK. Bonnema SJ. Brix TH. Hegedüs L. Risk of malignancy in thyroid incidentalomas detected by 18F-fluorodeoxyglucose positron emission tomography: a systematic review. Thyroid. 2012;22:918–925. doi: 10.1089/thy.2012.0005. [DOI] [PubMed] [Google Scholar]
- 7.Pagano L. Samà MT. Morani F. Prodam F. Rudoni M. Boldorini R. Valente G. Marzullo P. Baldelli R. Appetecchia M. Isidoro C. Aimaretti G. Thyroid incidentaloma identified by 18F-fluorodeoxyglucose positron emission tomography with CT (FDG-PET/CT): clinical and pathological relevance. Clin Endocrinol (Oxf ) 2011;75:528–534. doi: 10.1111/j.1365-2265.2011.04107.x. [DOI] [PubMed] [Google Scholar]
- 8.Kim BH. Na MA. Kim IJ. Kim SJ. Kim YK. Risk stratification and prediction of cancer of focal thyroid fluorodeoxyglucose uptake during cancer evaluation. Ann Nucl Med. 2010;24:721–728. doi: 10.1007/s12149-010-0414-6. [DOI] [PubMed] [Google Scholar]
- 9.Luciani A. Balducci L. Multiple primary malignancies. Semin Oncol. 2004;31:264–273. doi: 10.1053/j.seminoncol.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 10.Haberkorn U. Ziegler SI. Oberdorfer F. Trojan H. Haag D. Peschke P. Berger MR. Altmann A. van Kaick G. FDG uptake, tumor proliferation and expression of glycolysis associated genes in animal tumor models. Nucl Med Biol. 1994;21:827–834. doi: 10.1016/0969-8051(94)90162-7. [DOI] [PubMed] [Google Scholar]
- 11.Wang W. Larson SM. Fazzari M. Prognostic value of [18F] fluorodeoxyglucose positron emission tomographic scanning in patients with thyroid cancer. J Clin Endocrinol Metab. 2000;85:1107–1113. doi: 10.1210/jcem.85.3.6458. [DOI] [PubMed] [Google Scholar]
- 12.Van den Bruel A. Maes A. De Potter T. Mortelmans L. Drijkoningen M. Van Damme B. Delaere P. Bouillon R. Clinical relevance of thyroid fluorodeoxyglucose-whole body positron emission tomography incidentaloma. J Clin Endocrinol Metab. 2002;87:1517–1520. doi: 10.1210/jcem.87.4.8371. [DOI] [PubMed] [Google Scholar]
- 13.Schönberger J. Rüschoff J. Grimm D. Marienhagen J. Rümmele P. Meyringer R. Kossmehl P. Hofstaedter F. Eilles C. Glucose transporter 1 gene expression is related to thyroid neoplasms with an unfavorable prognosis: an mmunohistochemical study. Thyroid. 2002;12:747–754. doi: 10.1089/105072502760339307. [DOI] [PubMed] [Google Scholar]
- 14.Sandeep TC. Strachan MW. Reynolds RM. Brewster DH. Scélo G. Pukkala E. Hemminki K. Anderson A. Tracey E. Friis S. McBride ML. Kee-Seng C. Pompe-Kirn V. Kliewer EV. Tonita JM. Jonasson JG. Martos C. Boffetta P. Brennan P. Second primary cancers in thyroid cancer patients: a multinational record linkage study. J Clin Endocrinol Metab. 2006;91:1819–1825. doi: 10.1210/jc.2005-2009. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka H. Tsukuma H. Koyama H. Kinoshita Y. Kinoshita N. Kinoshita N. Oshima A. Second primary cancers following breast cancer in the Japanese female population. Jpn J Cancer Res. 2001;92:1–8. doi: 10.1111/j.1349-7006.2001.tb01040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Omür O. Ozcan Z. Yazici B. Akgün A. Oral A. Ozkiliç H. Multiple primary tumors in differentiated thyroid carcinoma and relationship to thyroid cancer outcome. Endocr J. 2008;55:365–372. doi: 10.1507/endocrj.k07e-058. [DOI] [PubMed] [Google Scholar]
- 17.Kaida H. Hiromatsu Y. Kurata S. Kawahara A. Hattori S. Taira T. Kobayashi M. Uchida M. Yamada K. Mihashi H. Umeno H. Kage M. Nakashima T. Hayabuchi N. Ishibashi M. Relationship between clinicopathological factors and fluorine-18-fluorodeoxyglucose uptake in patients with papillary thyroid cancer. Nucl Med Commun. 2011;32:690–698. doi: 10.1097/MNM.0b013e32834754f1. [DOI] [PubMed] [Google Scholar]
- 18.Park YW. Do IG. Park YK. Expression of the GLUT1 glucose transporter, p63 and p53 in thyroid carcinomas. Pathol Res Pract. 2006;202:759–765. doi: 10.1016/j.prp.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Grabellus F. Nagarajah J. Bockisch A. Schmid KW. Sheu SY. Glucose transporter 1 expression, tumor proliferation, and iodine/glucose uptake in thyroid cancer with emphasis on poorly differentiated thyroid carcinoma. Clin Nucl Med. 2012;37:121–127. doi: 10.1097/RLU.0b013e3182393599. [DOI] [PubMed] [Google Scholar]
- 20.Baek SK. Kwon SY. Woo JS. Jung KY. Kim GI. Prognostic Significance of COX-2 and VEGF-C Expression in Thyroid Papillary Carcinoma. Korean J Otolaryngol Head Neck Surg. 2004;47:1273–1277. [Google Scholar]


