Fig 1.
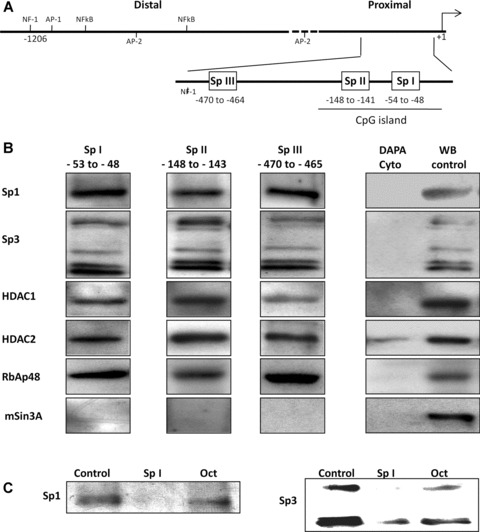
in vitro DAPA analysis indicates that the Sp I-III (GC) binding sites within the RIIα promoter bind multi-protein complexes containing Sp1, Sp3, HDACS 1 and 2 and RbAp48. (A) Schematic representation of the RIIα promoter region showing the putative transcriptional regulatory cis-elements. The transcriptional initiation site (+1) and the nucleotide position of the three Sp I-III (GC) binding elements in the proximal region are as shown. (B) DAPA analysis was performed with biotin labelled oligonucleotides for the individual Sp I-III (GC) sites and nuclear extracts from human myometrial cell cultures. Proteins which bound to the individual Sp1-III (GC) oligonucleotides were analysed by Western blotting using antibodies for Sp1, Sp3, HDAC1and 2, RbAp48 and mSin3A. All three Sp I-III (GC) elements bound protein complexes containing Sp1, Sp3, HDAC1 and 2 and RbAp48. mSin3A was not associated with the complexes, despite its presence in the myometrial cells. No binding was observed when the nuclear extracts were replaced with cytoplasmic extract (Cyto) except in the case of HDAC2 where a weak signal was observed. Western blotting of nuclear protein extracts (WB control 10 μg) were used as positive controls. Similar results were obtained in two further experiments. (C) Binding specificity of Sp1 and Sp3 to the Sp I (GC) element was determined by DAPA competition assays where 50× excess specific competitor (unlabelled Sp I oligonucleotide) or non-specific competitor (Oct element, see Table 1) was included in the DAPA binding reactions. Similar results were obtained for the Sp II and Sp III cis-elements within the RIIα promoter (data not shown).
