3.
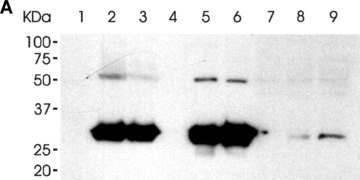
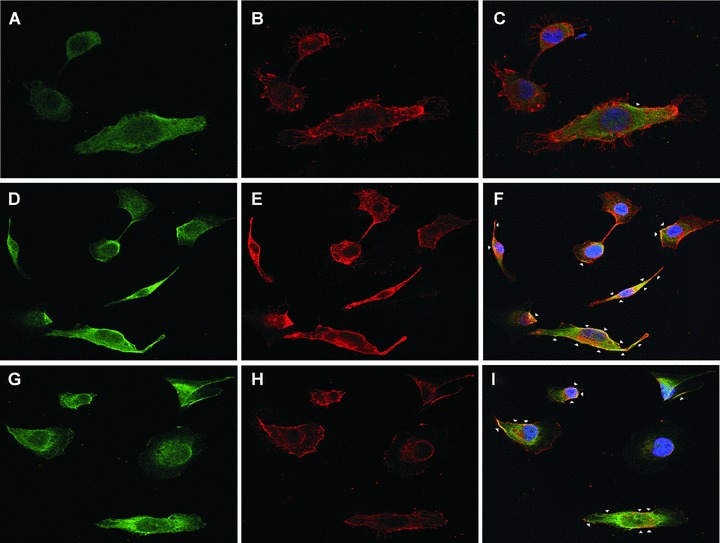
The binding of the LH3 N-terminal fragment and its glycosyltransferase-deficient counterpart on HT-1080 cell surfaces. (A) Western blot analysis after incubation with the hLH3N1 antibody: lanes 1–3 are cell pellets from untreated cells, DXD fragment treated cells and wild-type LH3 N-terminal fragment treated cells, respectively, demonstrating that both fragments (30 kD) bound to cell membranes; lanes 4–6 are medium samples purified on a Nickel column from the untreated cells, DXD fragment-treated cells and LH3 N-terminal fragment-treated cells, respectively, showing the same amount of the fragments (30 kD) used in all experiments. Lanes 7–9 are cell lysates purified on a Nickel column from untreated cells, DXD fragment treated cells and LH3 N-terminal fragment-treated cells, indicating trace amount of the fragments (30 kD) taken into the cells. (B) Immunofluorescence staining of non-permeabilized cells (A–C: untreated; D–F: DXD treatment; G–I: LH3N treatment) by the affinity-purified PLOD3 antibody (green colour, A, D and G), Wheat germ agglutinin (cell surface marker, red colour, B, E and H), and Hoechst 33258 (nuclei marker, blue colour). Parts C, F and I show the overlapping of the LH3 staining with the WGA staining on the cell surface (yellow colour, indicated by arrowheads). Untreated cells (C) are used as a background control, showing a small amount of endogenous LH3 on the cell surface. The cells treated with the DXD fragment (F) or the LH3N fragment (I) show much more overlap of the LH3 with the WGA, indicating that both fragments are bound to the cell surface.
