Fig. 1.
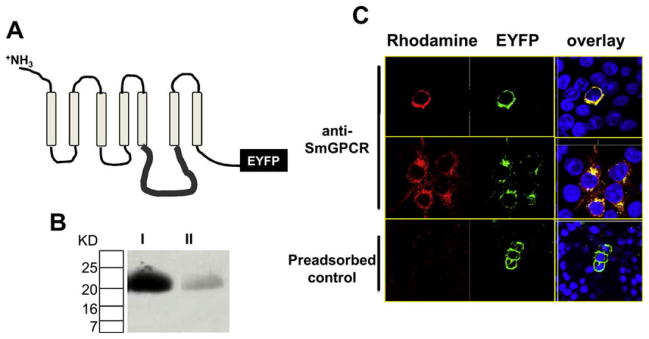
Production of an anti-SmGPCR polyclonal antibody. A portion of the third intracellular loop (il3) of SmGPCR (pos. 355–449) was expressed in E. coli as a recombinant His-tagged protein and purified to homogeneity prior to injecting into rabbits to produce anti-SmGPCR antibody (A) Schematic representation of SmGPCR showing a typical seven transmembrane topology and the il3 region targeted for antibody production. (B) Anti-SmGPCR antibody (lane I) or antibody that was pre-adsorbed with an excess of purified il3 antigen (Lane II) were tested against the purified il3 protein by Western blot analysis. The results show a strong, single immunoreactive band of the correct size in samples probed with anti-SmGPCR IgG but only a weak band in the pre-adsorbed control. (C) The antibody was tested first in HEK293E cells that were stably transfected with SmGPCR fused at the C-terminal end to EYFP. Cells were incubated with anti-SmGPCR antibody followed by a rhodamine-conjugated secondary antibody. An overlay of rhodamine (red) and EYFP (green) produced bright yellow fluorescence, indicating co-localization of the two signals. No rhodamine fluorescence could be detected in cells incubated with pre-immune serum (not shown) or anti-SmGPCR antibody that was pre-adsorbed with purified il3 antigen (pre-adsorbed control) Cells were counterstained with Dapi (blue) to visualize the nucleus. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
