Fig. 2.
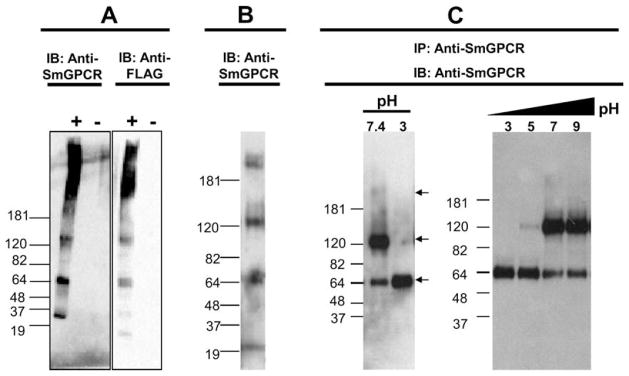
Western blot and immunoprecipitation analyses of SmGPCR. (A) HEK293E cells were transfected with a FLAG-tagged SmGPCR expressing vector (+) or empty vector (−). Cells were homogenized and aliquots of a solubilized crude membrane fraction were immunoblotted either with anti-SmGPCR IgG or with a monoclonal anti-FLAG antibody. The sizes of the protein ladder are indicated. (B) A representative Western blot analysis of an adult S. mansoni extract probed with anti-SmGPCR antibody. (C) SmGPCR was immunoprecipitated from a crude extract of S. mansoni, using beads covalently coupled to anti-SmGPCR IgG and then immunoblotted with the same antibody. The receptor was eluted from the antibody beads under acidic conditions and immediately neutralized to pH 7.5. At neutral pH we see three bands corresponding to the monomer (65 kDa), dimer (130 kDa) and a faint high MW species (>180 kDa) but only the monomer can be detected when the sampled was acidified to pH 3 prior to immunoblotting (left panel). A stepwise increase in pH (pH 3–9) caused progressive dimerization of the receptor (right panel). IB, immunoblotted; IP, immunoprecipitated.
