fig 6.
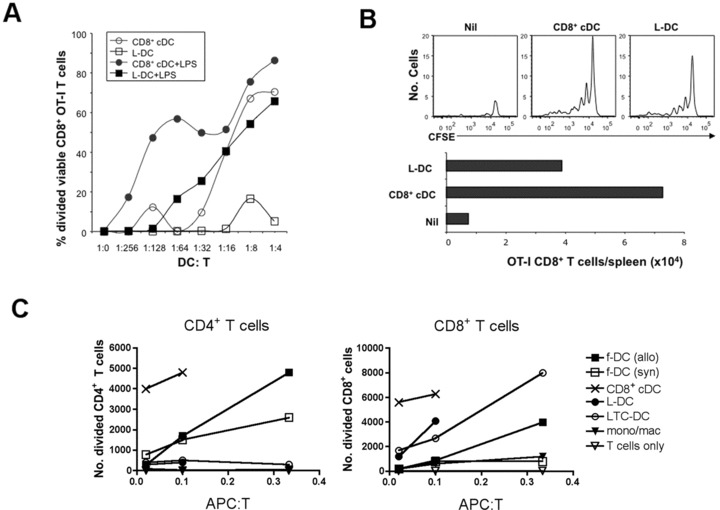
Antigen-presenting capacity of L-DC. CD8α+ cDC, myeloid cells (mono/mac) and L-DC subsets were sorted from C57BL/6J spleen based on gating protocols described in Figure 4. LTC-DC were collected as non-adherent cells from LTC established from spleens of B6.SJL mice. f-DC were enriched from spleens of C57BL/6J and CBA/H mice using CD11c+ microbead positive selection (MACS). (A) To measure in vitro cross-presenting ability, L-DC and CD8α+ cDC were pulsed with OVA (10 μg/ml) and co-cultured with purified CFSE-labelled CD8+ T cells (105) from OT-I mice ±LPS (1 μg/ml). Proliferation of cells was measured by dilution of CFSE after 4 days for calculation of percentage divided cells. (B) Isolated L-DC and CD8α+ cDC were pulsed with OVA (as in A), and 104 cells injected i.v. into B6.SJL (CD45.1) mice previously injected with 105 CFSE-labelled OT-I lymphocytes. Spleens were harvested at 34 days following rechallenge of mice at 25 days with OVA (10 μg, i.v.), and assessed flow cytometrically for CFSE and total number of OT-I CD8+ T cells in spleen. (C) L-DC and LTC-DC were compared with APC subsets isolated from spleen for capacity to induce a MLR. Responder T cells were prepared from CBA/H mice by depletion of B cells, myeloid cells and DC using magnetic beads, labelled with CFSE, and co-cultured with APC, using T cells only as a control. Responding T cells were analysed flow cytometrically at 4 days following antibody staining to gate live (PI−), CD11c−, CD4+ or CD8+ populations. Number of divided cells reflects cells showing a reduction in CFSE intensity.
