Figure 4.
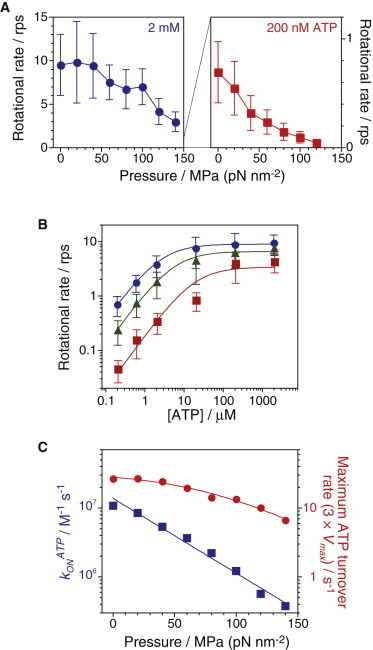
Rotational rate of wild-type F1-ATPase. (A) Pressure dependence of rotational rates under saturating (2 mM, n = 12–43, total = 173) and limiting ATP concentrations (200 nM, n = 38–75, total = 406) (mean ± SD). (B) Rotational rates (mean ± SD) as a function of ATP concentration at 0.1 (circles, n = 22–81, total = 314), 60 (triangles, n = 12–102, total = 294), and 120 MPa (squares, n = 25–79, total = 283). Curves show the fitting line with the Michaelis-Menten equation, V = Vmax × [ATP]/(Km + [ATP]); Vmax = 8.9 ± 0.3, 6.5 ± 0.5, and 3.4 ±1.1 s−1; Km = 2.4 ± 0.1, 5.3 ± 0.6, and 18 ± 8 μM at 0.1, 60, and 120 MPa, respectively. (C) Pressure dependence of the apparent ATP binding rate, konATP (squares) and maximum ATP turnover rate, 3 × Vmax (circles). The plots of konATP were fitted by Eq. 1 with konATP(0.1) = 14 ± 2 ×106 M−1 s−1 and ΔV‡ = +100 ± 10 Å3. The plots of 3 × Vmax were fitted by Eq. 2 with kp-ind = 33 ± 5 s−1, k0 = 180 ± 90 s−1 and ΔV‡ = +88 ± 15 Å3. To see this figure in color, go online.
