Abstract
Young children are at increased risk for valproic acid (VPA) hepatotoxicity. Urinary organic acid profiles, as a measure of mitochondrial function, were obtained in children 3.5 to 17.3 years old treated for seizure disorders with valproic acid (VPA; n=52). Age-matched patients treated with carbamazepine (CBZ; n=50) and untreated healthy children (n=22) served as controls. Age-related changes in organic acid profiles were observed in all three groups. Although untreated and CBZ control subjects were not distinguished by the principal component analysis (PCA) scores plot, a distinct boundary was apparent between the VPA and control/CBZ groups. Inter-individual variability in VPA-induced alterations in endogenous pathways reflecting branched chain amino acid metabolism and oxidative stress was observed. The data suggest that more detailed metabolomic analysis may provide novel insights into biological mechanisms and predictive biomarkers for children at highest risk for serious toxicity.
INTRODUCTION
Valproic acid (VPA), a widely prescribed antiepileptic drug, is associated with a severe idiosyncratic hepatotoxicity characterized by microvesicular steatosis and necrosis. Although rare, this toxicity can be fatal particularly in children < 2 years,, those with developmental delays or metabolic disorders (especially disorders of mitochondrial function), and those concurrently receiving enzyme-inducing medication (1–3). While the exact mechanism of VPA-induced hepatotoxicity has not been definitively identified, is hypothesized that this effect is mediated through interference with mitochondrial β-oxidation. Competitive inhibition of β-oxidation enzymes (4, 5) and depletion of carnitine (6), coenzyme A (4, 5, 7) and glutathione (8) stores during VPA metabolism may impair lipid metabolism, resulting in steatosis. Additionally, oxidative stress (9, 10) may contribute to the toxic effects of VPA.
Valproate is a substrate for branched-chain amino acid metabolism (4), utilizing the same enzymes and cofactors needed for mitochondrial lipid metabolism. Due to its small size, VPA is thought to passively diffuse across the mitochondrial outer membrane independent of the carnitine shuttle (4, 5). Once inside the mitochondrial matrix, VPA is converted to valproyl-CoA, a substrate for dehydrogenation by 2-methyl-branched chain acyl-CoA dehydrogenase, forming 2-ene-VPA-CoA (5). The 2-ene-VPA-CoA is converted to 3-OH-VPA-CoA by the β-oxidation enzyme enoyl-CoA hydratase, which is then converted to 3-keto-VPA-CoA by an unidentified membrane-bound NAD+-dependent dehydrogenase (5). Approximately 40% of the 3-keto-VPA-CoA is cleaved into propionyl-CoA and pentanoyl-CoA by an unidentified thiolase with the remaining 60% likely hydrolyzed to 3-keto-VPA (11).
Several studies investigating the metabolic consequences of VPA have been conducted using supratherapeutic doses in rodents (12–14). Although the results of these studies uniformly demonstrate alterations in metabolic endpoints, no unifying mechanism of hepatotoxicity has been presented In one study using 13C-labeled glucose, VPA caused a simultaneous decline in liver glycogen turnover and ribose production without alteration of glucose uptake or metabolism (13). In another metabolic profiling study, VPA increased urinary glucose over time post-dose, and altered proteins involved in glycogenolysis (14). This observation is consistent with in vitro studies using isolated rat liver mitochondria that demonstrated a significant inhibition of pyruvate uptake across the mitochondrial membrane by VPA and its metabolites (15), along with diminished rates of ATP synthesis fueled by pyruvate (16). Efforts to understand the mechanisms by which VPA alters cellular metabolism has implications not only for the hepatotoxicity, but also weight gain, a common side effect of VPA therapy thought to be related to increased availability of long-chain fatty acids (17, 18).
Attempts to identify the specific factor(s) placing young children at increased risk for VPA hepatotoxicity have focused on the pathways of VPA biotransformation with particular interest in those that differ substantially between children and adults, thus providing insights into the mechanisms leading to preferential toxicity in susceptible children. A common, but not universal, finding is a role for 2-n-propyl-4-pentenoic acid (4-ene-VPA) (19–24), a potentially hepatotoxic metabolite formed by ω-1 oxidation of VPA (25). Although it has been suggested that 4-ene-VPA formation is increased in younger children (23), age-related differences have not been reproducible (20, 24). However, thiol conjugates of VPA metabolites potentially reflect the burden of reactive metabolites formed from VPA, and have been reported to decline with increasing age in childhood (26). In a comprehensive analysis of VPA metabolites in 91 children receiving VPA mono- and polytherapy, we were unable to replicate any age-related changes in urinary VPA metabolite concentrations when developmental changes in urinary creatinine concentration were taken into consideration (manuscript submitted for review). Consequently, the current investigation pursues an alternative hypothesis that developmental differences in mitochondrial function exist, and that introduction of a medium chain fatty acid load (e.g., VPA) may cause age-dependent perturbations in mitochondrial function, as measured by urinary organic acid profiles.
RESULTS
Subjects
This study involved 127 children ages 1.7–17.6 years. Subject demographics are presented in Table 1.
Table 1.
Demographic information for the study cohort.
| Average ± SD (Range) | |||
|---|---|---|---|
|
| |||
| Characteristic | CBZ | VPA | No Meds |
| Gender | M: 34 | M: 34 | M: 11 |
| F: 16 | F: 18 | F: 11 | |
| Age, y | 10.4 ± 4.1 (1.7–17.6) | 10.2 ± 3.9 (1.9–17.3) | 11.1 ± 4.9 (3.0–17.3) |
| Weight, kg | 40.7 ± 21.3 (11.2–99.1) | 33.8 ± 15.8 (7.6–86.4) | 41.4 ± 17.8 (13.9–67.9) |
| Race1 | AA: 4 | ||
| AA: 1 | AS: 1 | ||
| C: 42 | C: 41 | AA: 4 | |
| H: 4 | H: 4 | C: 18 | |
| AA/C: 3 | Oth: 1 | ||
| AA/C: 1 | |||
| Dose, mg/kg/day | 16.6 ± 10.1 (3.1–45.8) | 25.9 ± 18.7 (7.6–138.9) | N/A |
AA = African American, AS = Asian, C = Caucasian, H = Hispanic, Oth = Other, AA/C = African American/Caucasian.
Effects of sample and subject age on organic acid profiles
Concentrations of urinary organic acids determined in this study were comparable to values reported in Swiss, Turkish and American pediatric populations (27–29). However, because these data were generated from a set of residual urine samples, the effect of sample age (storage time) on organic acid profiles was assessed. Literature indicates that lactic, 2-hydroxyglutaric, 2-ketoglutaric, succinic, 3-hydroxypropionic and hippuric acids are likely to change as a result of bacterial contamination of the urine sample (30). A regression was performed between sample age and individual organic acid concentrations. Seven organic acids were identified as having significant nonzero correlations to sample age (p<0.05): 2-ketoisocaproic, succinic, methylsuccinic, aconitic, isocitric, methylcitric and 4-hydroxyphenylpyruvic acids. None of these correlations retained significance using a Bonferroni-corrected β level of 0.0007 (α=0.05/n=70). Removing the organic acids that were most strongly correlated with sample age did not appreciably alter the principal component model described in more detail below, suggesting that sample storage contributed little variance to the data set.
Prior to assessing VPA effects on organic acid profiles, the underlying effects of subject age in the data set were determined. Normalization of the data set was shown to introduce some age correlation structure. All organic acids were reported as mmol acid/mol creatinine to account for variation in urine dilution. However, this normalization can be problematic in pediatric samples, where expected creatinine excretion increases with age (31). Creatinine showed a significant correlation to subject age (r=0.383; r2=0.145; P=0.0061. Excretion rate of creatinine did not differ significantly between CBZ and VPA subjects, indicating that these drugs do not alter creatinine excretion, as previously published (32).
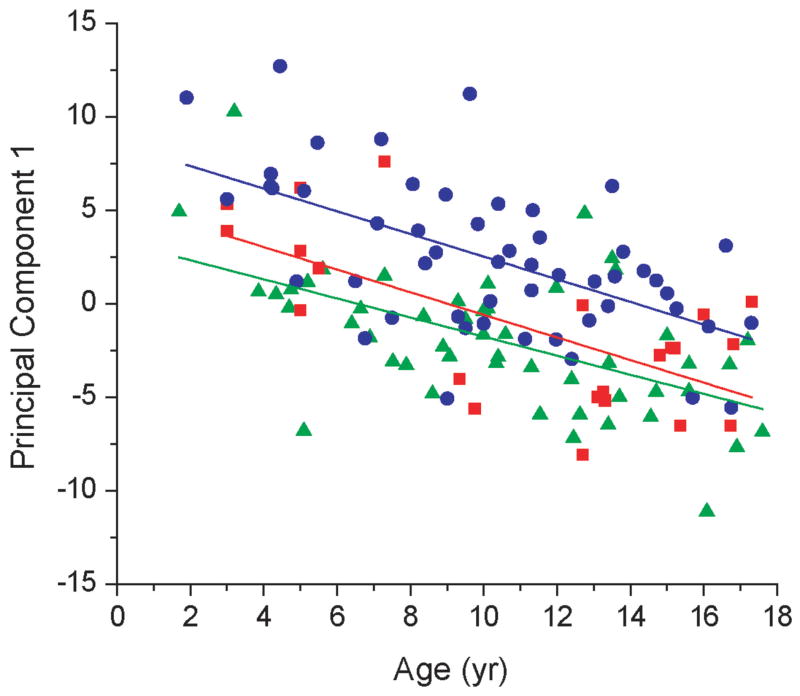
A principal component model was generated, and the principal components were then regressed against age. The first principal component (PC1) accounted for 31.2% of the variance and showed a highly significant age trend (p<0.0001) with an r value of −0.55. PC1 was primarily defined by 2-hydroxyglutaric, 3-hydroxyisobutyric, 3-hydroxyisovaleric and glutaric acids. All organic acids were negatively correlated with age, signifying that their rate of change was invariant with age, or was slower than the developmental increase in creatinine. When PC1 was regressed with subject age, the trends were parallel for the VPA and control groups (Figure 1). These results indicate that age-related changes in urinary organic acid profiles were preserved in all three groups, and further imply that this relationship was not affected by drug administration.
Figure 1.
Age trends identified by principal components analysis (PCA). Subject age was regressed against PC1 scores separately for each group: Control (
 ), CBZ (
), CBZ (
 ), and VPA (
), and VPA (
 ). The lines of best fit for each group are colored according to the color of the symbol for that group. The similarity in the slopes of the regressed lines indicates that age-related changes in organic acid profiles were similar in each group, and independent of drug treatment.
). The lines of best fit for each group are colored according to the color of the symbol for that group. The similarity in the slopes of the regressed lines indicates that age-related changes in organic acid profiles were similar in each group, and independent of drug treatment.
Effect of drug administration on organic acid profiles
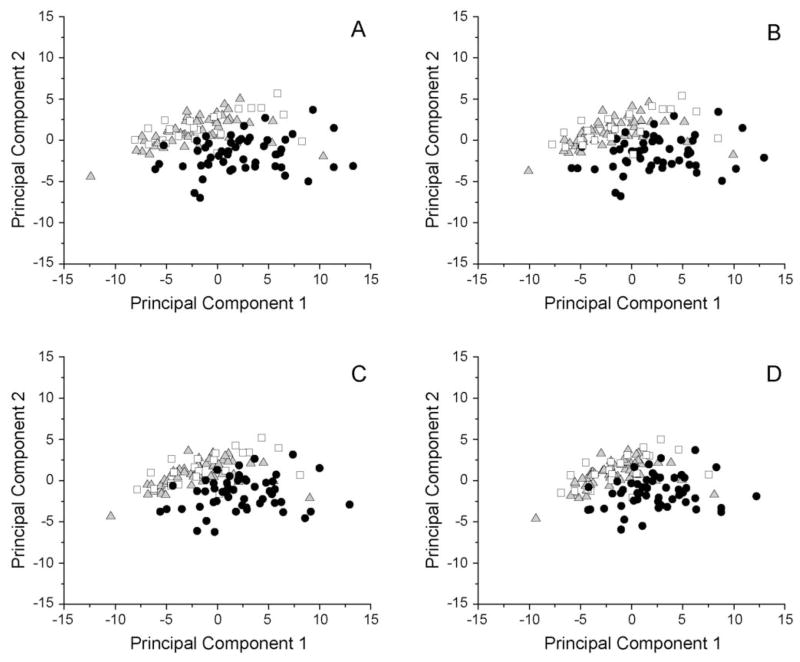
In the PCA model described above, the first three components explained 44.4% of the variation in the data set, with PC1 explaining 31.2% of the variance. The scores plot is presented in Figure 2A. Control and CBZ samples overlapped completely and were mostly confined to a cluster along PC2. There was a distinct boundary between the control/CBZ and VPA groups, with the VPA subjects showing interindividual variability in the extent to which individual samples deviated from the control/CBZ grouping; an age trend was identified along PC1.
Figure 2.
Principal components analysis scores plot. (A) Principal components analysis (PCA) was performed on the data correlation matrix to uncover the major sources of variation in the dataset. The first principal component (PC1) accounts for 31.2% of the variance, and is primarily defined by 2-hydroxyglutaric, 3-hydroxyisobutyric, 3-hydroxyisovaleric and glutaric acids. PC2 is primarily defined by an inverse relationship between uracil and 4-hydroxyphenylpyruvic, 2-ketoisovaleric, acetoacetic, 2-hydroxybutyric, 2-keto-3-methylvaleric and 2-ketoisocaproic acids. The Control (□) and CBZ (
 ) groups were superimposable, whereas a distinct boundary is observed between the control/CBZ and VPA (●) groups, with the VPA subjects showing interindividual variability in the extent to which individual samples deviate from the control/CBZ grouping. To address the possibility that observed changes attributed to VPA were due to organic acids that may have been derived from VPA metabolism, organic acids exceeding specified correlation thresholds with any VPA metabolite were successively excluded from the PCA analysis. The threshold was set at R2 = 0.5 (B), and sequentially reduced to thresholds of 0.4 (C) and 0.3 (D), and the distribution of values compared to the original scores plot containing the full data set (A). Data were labeled by group (□, control;
) groups were superimposable, whereas a distinct boundary is observed between the control/CBZ and VPA (●) groups, with the VPA subjects showing interindividual variability in the extent to which individual samples deviate from the control/CBZ grouping. To address the possibility that observed changes attributed to VPA were due to organic acids that may have been derived from VPA metabolism, organic acids exceeding specified correlation thresholds with any VPA metabolite were successively excluded from the PCA analysis. The threshold was set at R2 = 0.5 (B), and sequentially reduced to thresholds of 0.4 (C) and 0.3 (D), and the distribution of values compared to the original scores plot containing the full data set (A). Data were labeled by group (□, control;
 , CBZ; ●, VPA)
, CBZ; ●, VPA)
As VPA is an 8-carbon medium chain fatty acid we considered the possibility that the observed difference in organic acid profiles between VPA and the other two groups was due to the formation of “endogenous” organic acids derived from metabolism of VPA or further metabolism of its primary metabolites. Principal components analysis was repeated excluding any organic acids that correlated with VPA metabolites exceeding a threshold of r2=0.5 (8 organic acids; Figure 2B), r2=0.4 (15 organic acids; Figure 2C), or r2=0.3 (24 organic acids; Figure 2D). Removal of these organic acids did not result in any demonstrable change in the plots relative to Figure 2A, and the distinct boundary between VPA and control/CBZ samples persisted in all plots. Thus, intercorrelations in the VPA metabolites and organic acids had little bearing on the calculation of the initial VPA PCA model, lending credibility to the model robustness and the observed trends.
PCA could not distinguish between healthy control subjects and those receiving CBZ. Thus, the organic acids identified as significant on the PC2 axis by this model relate solely to VPA-induced changes in metabolism. PC2 is primarily defined by an inverse relationship between uracil and 4-hydroxyphenylpyruvic, 2-ketoisovaleric, acetoacetic, 2-hydroxybutyric, 2-keto-3-methylvaleric and 2-ketoisocaproic acids. These organic acids provide insights into the metabolic pathways uniquely perturbed by VPA. 2-Keto-3-methylvaleric and 2-ketoisovaleric acids are intermediates in branched-chain amino acid metabolism, which is known to be altered by VPA, and are elevated in lactic acidosis and ketoacidosis. 2-Hydroxybutyric acid, as a byproduct of glutathione synthesis, is a marker of oxidative stress, also a known effect of VPA. 2-Keto-3-methylvaleric acid is generated by L-leucine metabolism and is found in very high levels in Maple Syrup Urine Disease, but has not been reported to be altered by VPA therapy.
Other more subtle trends were noted in the PC scores plot. When the first three principal components were regressed against demographic variables, PC1 showed a highly significant (p<0.0001) correlation to age, group, weight and dose. A table of p values for PC1 regressed to various demographic variables is shown in Table 2, representing regression for the data as a whole and broken out into individual groups. PC2 was significantly correlated to age, group, race, and weight (Table 3). PC3 was significantly correlated to group and the presence of concurrent medications classified (for VPA subjects) as monotherapy, non-inducer medications or enzyme inducing medications (Table 4).
Table 2.
P values for the regression of Principal Component 1a against various demographic variables, using both the entire data set and the data set broken out into the three groups (CBZ, VPA and control). Significant correlations are noted by bold type.
| Variable | All | CBZ | Control | VPA |
|---|---|---|---|---|
| Group | <0.0001 | - | - | - |
| Age | <0.0001 | <0.0001 | 0.0005 | <0.0001 |
| Gender | 0.7373 | 0.9514 | 0.5308 | 0.8603 |
| Race | 0.6576 | 0.9027 | 0.5130 | 0.1453 |
| Weight | <0.0001 | 0.0003 | 0.0002 | <0.0001 |
| Dose | <0.0001 | 0.1121 | - | 0.0019 |
| Concurrent Medications | - | - | - | 0.5339 |
Principal Component 1 represents the linear combination of factors that accounts for the largest amount of variability in the data, and each succeeding component (PC2, PC3) has the highest variance possible under the constraint that it is uncorrelated with the preceding components. PC1 accounted for 31.2% of the variability in the current dataset.
Table 3.
P values for the regression of Principal Component 2 against various demographic variables, formatted as described in Table 2.
| Variable | All | CBZ | Control | VPA |
|---|---|---|---|---|
| Group | <0.0001 | - | - | - |
| Age | 0.0002 | <0.0001 | 0.0039 | 0.0125 |
| Gender | 0.8431 | 0.2905 | 0.4777 | 0.6490 |
| Race | 0.0380 | 0.2142 | 0.3559 | 0.1964 |
| Weight | 0.0069 | 0.0003 | 0.0040 | 0.0113 |
| Dose | 0.3045 | 0.2674 | - | 0.6031 |
| Concurrent Medications | - | - | - | 0.6926 |
Table 4.
P values for the regression of Principal Component 3 against various demographic variables, formatted as described in Table 2
| Variable | All | CBZ | Control | VPA |
|---|---|---|---|---|
| Group | 0.0239 | - | - | - |
| Age | 0.7877 | 0.5544 | 0.2582 | 0.8455 |
| Gender | 0.3792 | 0.0640 | 0.2715 | 0.9151 |
| Race | 0.6321 | 0.4677 | 0.6304 | 0.9294 |
| Weight | 0.9032 | 0.4108 | 0.6008 | 0.5823 |
| Dose | 0.7792 | 0.5114 | - | 0.5942 |
| Concurrent Medications | - | - | - | 0.0057 |
Differential effect of VPA and CBZ on organic acid profiles
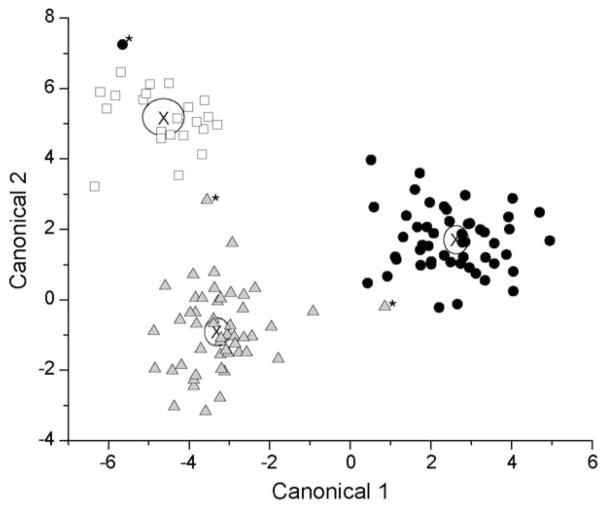
The CBZ group serves as a control for the potential effect of seizure disorders on organic acid profiles. Comparison of the two groups by PCA identified a significant effect of VPA on urinary organic acid profiles in children, but did not show an effect from CBZ. Subsequently, the data were modeled using linear discriminant analysis, and a unique metabolic effect from both VPA and CBZ was noted. A significant two canonical variable model was calculated from the data, with canonical coefficient 1 (CC1) accounting for 66.9% of the data variation and CC2 accounting for the remaining 33.1%. Misclassification rate was 2.4%, with 3 of the 124 samples misclassified – one CBZ sample was misclassified as a VPA sample, and two samples (one CBZ and one VPA) were classified as Control samples. Differentiation between group means was highly significant, as gauged by the Wilks’ Lambda test (p<0.0001). The canonical plot is shown in Figure 3. Note that in this model, the CBZ and control samples were distinguishable, as compared to the PCA model presented above. This suggests that CBZ perturbs metabolism in a mechanism distinct from VPA. As PCA did not detect this difference, the perturbation from CBZ is likely more subtle.
Figure 3.
Linear discriminant analysis of urinary organic acids by study group. Control (□), CBZ (
 ), and VPA (●) group means were significantly discriminated with a Wilks’ Lambda test (p<0.0001) and a 2.4% misclassification rate. Misclassified samples were labeled by *. “X” denotes the center of each cluster.
), and VPA (●) group means were significantly discriminated with a Wilks’ Lambda test (p<0.0001) and a 2.4% misclassification rate. Misclassified samples were labeled by *. “X” denotes the center of each cluster.
On examination of the canonical plot, canonical coefficient 1 separated VPA samples from the control and CBZ samples. CC1 is primarily defined as a contrast axis between pimelic, 2-hydroxyglutaric, 4-hydroxyphenylpyruvic and succinic acids against glycolic, azelaic and 3-methylglutaric acids. Five of these (pimelic, 2-hydroxyglutaric, succinic, azelaic and 3-methylglutaric acids) are dicarboxylic acids known to be excreted in larger quantities under conditions of oxidative stress and impaired fatty acid oxidation. Canonical coefficient 2 separated the CBZ samples from the VPA and control groups (with some separation of the VPA and control samples also noted). CC2 is primarily defined by a contrast between citric, 3-hydroxyisobutyric and glutaric acids and aconitic and pimelic acids. Increased excretion of dicarboxylic acids implies increased oxidative stress, and intermediates in the Krebs cycle also appear to be altered.
Age-dependent perturbation of organic acid profiles in patients treated with VPA and CBZ
Differential metabolic perturbation by age in the CBZ and VPA groups was noted. Organic acid levels were regressed to age for each group (VPA, CBZ or control) and results were compared among groups. Eight organic acids were identified as having significant nonzero age trends in the VPA group alone: pyruvic (R=−0.49), ethylmalonic (R=−0.51), 5-hydroxyhexanoic (R=−0.31), suberic (R=−0.37), sebacic (R=−0.38), and hippuric (R=−0.33) acids, as well as isovalerylglycine (R=−0.30). As these were not significant for the other two groups, these are potential organic acid biomarkers of age-dependent VPA effects on mitochondrial function. CBZ also showed some significant nonzero age trends in 3-hydroxyisobutyric (R=−0.32), 4-hydroxybutyric (R=0.30), adipic (R=−0.50) and 2-ketoglutaric (R=−0.39) acids, potential markers of oxidative stress.
DISCUSSION
The analyses presented in this report represent a new approach to the characterization of age-related differences in VPA effects on a biological system. In the past, attention has focused on a terminal olefin metabolite of VPA, 4-ene-VPA, due to its structural similarity to known hepatotoxins, such as 4-pentenoic acid. Kondo et al. reported that 4-ene-VPA formation is increased in younger children and declines with increasing age (23), but these age-related differences have not been replicated by others (20, 24). Evidence for increased bioactivation of VPA in younger children has been presented by Gopaul et al. on the basis of increased urinary concentrations of N-acetylcysteine conjugates of (E)-2,4-diene VPA, reflecting detoxication of reactive VPA metabolites by glutathione conjugation (26).
In urinary metabolite studies it is common practice to apply a correction factor using urinary creatinine concentrations to adjust for the effects of hydration status on observed drug and metabolite concentrations. However, creatinine production increases with age between birth and adolescence with the acquisition of muscle mass, and is affected by gestational age in newborns and anorexia in adolescents (summarized in (33)). Thus, in a comprehensive analysis of VPA metabolite profiles in 91 children receiving VPA both as monotherapy and polytherapy, we were unable to replicate any age-related changes in urinary VPA metabolite concentrations when developmental changes in urinary creatinine concentration were taken into consideration (manuscript submitted for review). We therefore considered that changes in body composition characteristic of growth and development in children are likely associated with increased metabolic demands. Given that impairment of mitochondrial β-oxidation has been implicated in VPA hepatotoxicity, and developmental changes in mitochondrial function may be implied by the age-dependent changes in normal ranges for urinary organic acid concentrations used in pediatric settings to diagnosis inborn errors of metabolism, we hypothesized that the developmental context in which VPA is administered may be an important determinant for age-related differences in the risk of serious forms of toxicity. The data presented in this study represent an initial exploration of this alternative approach.
There are few, if any, studies using comprehensive profiling of urinary organic acids or other biomarkers to assess alterations to mitochondrial metabolism by VPA on a population basis in children. The data presented in this study provide a unique opportunity to examine the variability in endogenous metabolic consequences of VPA in children, and to formulate hypotheses for further prospective studies. Data models revealed that a systematic metabolic change occurred in response to VPA treatment in children without overt hepatic damage, although significant interindividual variability in metabolic responses to VPA was observed. A final key finding was an age-dependent metabolic response to VPA, thus giving a new perspective on the unexplained VPA toxicity in young children to guide future investigation. Because of the retrospective analysis of residual urine samples, interpretation is limited to the identification and description of potentially interesting metabolic changes and issues related to pediatric metabolic profiling, subject to validation in future prospective investigations.
Age trends are a significant issue in the metabolic profiling of pediatric populations. As discussed above creatinine excretion changes with age, but is commonly used to account for fluctuations in urine concentration between samples. Thus, correction for urinary creatinine concentration typically introduces an age trend into the data (if one was not already present). Furthermore, those involved in the medical treatment of young children are well aware that the range of normal values for individual organic acids changes with increasing postnatal age, despite these values routinely being reported relative to urinary creatinine. The challenge in studies of this type is to differentiate changes due to the intervention from the background noise generated by increasing age, particularly if the factor of interest has its own age dependency. An important finding in this study is that organic acid profiles changed with time, and this change was independent of drug administration. To the extent that individual organic acids can serve as surrogate biochemical markers of mitochondrial dysfunction, the data raise the possibility of a mitochondrial “phenotype”, and that this phenotype changes with age. A major challenge will be to refine assessment of mitochondrial phenotype to determine the phenotype (i.e., at a younger age) most susceptible to toxicity, and thus provide testable hypotheses for the mechanisms of toxicity.
A differential effect of VPA on organic acids was noted in the PCA model, as illustrated from the varying distance of VPA subjects to the control/CBZ cluster. These metabolic changes are drug-specific, but not disease-specific, as CBZ subjects serving as the disease control do not differ from the healthy controls. Furthermore, an age-dependent metabolic response to VPA was observed. Both PCA and discriminant analysis showed deviation of the VPA metabolic profiles from the control and CBZ groups. Clustering revealed groupings of VPA subjects according to age, and identified two subsets of young subjects within the VPA group. Finally, it is notable that there is considerable inter-individual variability in the extent to which individual patients deviate from the control/CBZ cluster, with some VPA patients indistinguishable from the control and CBZ groups while others deviate considerably. Taken together, one can view VPA administration in children as perturbation of a dynamic system with a medium chain fatty acid load, and that consequences in terms of mitochondrial function may change significantly throughout childhood. In this context, it would not be unreasonable to observe inter-individual variability in the ability to respond to that perturbation across a treated population with, perhaps, more limited ability to adapt at discrete ages/developmental stages.
A major limitation of this study is its design. The goal of the original investigation was to characterize population variability in the patterns of VPA biotransformation and the effect of age on those profiles. This report represents a change in perspective from “what children’s developing systems do to the drug” to “what the drug does to children’s developing systems”, and necessarily involved secondary analysis of the samples to address that change in perspective. All subjects in this study were deemed healthy by standard laboratory measures and so it was not possible to address specifically the relationship between organic acid profiles and hepatotoxicity. Also, the study was designed to collect urine under steady state conditions after patients had been stabilized on their doses of VPA and CBZ. Thus, it was not possible to assess the extent to which organic acid profiles changed following VPA treatment. Nevertheless, the insights gained provide valuable insights and testable hypotheses for future studies.
An additional limitation is the scope of analytes used to assess metabolic effects of VPA. Urinary organic acid profiles are routinely available in tertiary care pediatric settings, and were used in this investigation as a surrogate measure of metabolic function and, potentially, mitochondrial function. Expanding the repertoire of analytes to interrogate the response of a broader complement of biological pathways to VPA administration, analogous to the metabolomic approaches that have been applied in both animal (34) and human (35) studies to identify endogenous profiles predictive of acetaminophen-induced hepatotoxicity, has the potential to elucidate biological mechanisms and identify sensitive and specific biomarkers for toxicity, especially those predictive of children at highest risk for serious toxicity.
Overall, this study indicates that human pediatric metabolic profiling of individual response to VPA provides a new perspective for investigating the mechanistic basis of age-related susceptibility to VPA hepatotoxicity. Clearly, prospective investigation is needed to more precisely identify the factors contributing to variability in VPA metabolic response. Longitudinal studies designed to better identify individual toxicity risk by examining VPA metabolic response over time are needed.
METHODS
Subjects
A retrospective analysis was conducted on residual urine samples from two separate studies. The first was a study of children receiving CBZ (n=50) or VPA (n=52) for routine management of seizure disorders. CBZ and VPA subjects were recruited at Children’s Mercy Hospitals and Clinics (Kansas City, MO), Kosair Children’s Hospital (Louisville, KY) and Primary Children’s Medical Center (Salt Lake City, UT), and were age- and gender-matched. For the original study, children of both genders between 1 and 16 years of age stably maintained on VPA or CBZ for at least two weeks, either as monotherapy or in conjunction with other antiepileptic agents as determined by their primary treating physician, and without evidence of clinical hepatic or renal dysfunction were eligible. Exclusion criteria included: 1) any clinical contraindication preventing collection of a urine sample (≥ 5 ml) during routine health visits; and 2) non-compliance with VPA or CBZ therapy during the 24 hours prior to sample collection. Urine samples were collected over one complete steady state dosing interval of VPA or CBZ. On the day prior to a scheduled clinic visit, participants were instructed to spontaneously empty their bladder prior to taking their last daily dosage of VPA. All urine produced overnight (generally 8 to 12 hours) was subsequently collected in provided containers until the next (morning) dose of medication was taken. After collection, urine containers were either brought to the clinic visit or were recovered from the homes by study personnel.
A comparison group of healthy children receiving no medications and with no underlying medical conditions (n=22) participating in two phenotyping studies investigating the ontogeny of CYP2D6 at Children’s Mercy Hospitals and Clinics were selected to span the age distribution of the CBZ and VPA subjects. In children aged 2 to 5 years, an overnight collection (as described for the VPA/CBZ study) was used, whereas a 4-hour collection interval was used for children >5 years. All original studies were approved by the Institutional Review Boards at each participating institution; use of residual samples for the current study was approved by the Children’s Mercy Hospital Pediatric Institutional Review Board, and ratified by the IRBs at the partner institutions. Written informed consent was obtained from parents/guardians, and assent from the patients, when applicable, prior to participation in the study. This study was designated as Protocol #10606 within the Eunice Kennedy Shriver National Institute of Child Health and Human Development Network of Pediatric Pharmacology Research Units (PPRU), and registered as Study NCT00224952 at ClinicalTrials.gov.
Analysis of Organic Acids
Organic acid analysis was conducted by the Biochemical Genetics Laboratory at Children’s Mercy Hospitals and Clinics according to the Standard Operating Procedure established in lab, and based on the methods of Tanaka et al (36) and Sweetman et al (37). Samples were stored at −20°C or below until gas chromatography/mass spectrometry (GC/MS) analysis. Prior to analysis, the creatinine concentration was determined for each urine sample using the Jaffe method. A volume of urine containing 1 μmol creatinine was then diluted to 2 mL with water. Tropic and ketocaproic acid were added as internal standards. Solutions were alkalinized with hydroxylamine and NaOH and heated at 60°C for 30 minutes. The reaction was stopped with cooling and the solution was acidified with 6 N HCl. Organic acids were extracted with 2 × 2 mL volumes of ethyl acetate, which were dried at 30°C under nitrogen. Derivatization was performed using 150 μL N,O-Bis(trimethylsilyl)trifluoroacetamide (BSTFA):pyridine (2:1 v:v). The mixture was heated at 65°C for 30 min.
All analyses were performed by GC/MS using a Agilent Technologies (Santa Clara, CA) 5890 Series II GC coupled to a HP 5972 MSD. A 30 meter Phenomenex Zebron ZB-1 (100% dimethylpolysiloxane) capillary column was utilized, of dimensions 0.25 mm i.d. with 0.25 μm stationary film thickness. Injection volume was 1 μL. The initial temperature of the column oven was 60°C. This was increased to 280°C at 7°C/min and held for 3 min. The injector temperature was 250°C and the transfer line temperature was 280°C. Helium carrier gas was maintained at a pressure of 0.5 psi. Reported results are limited to a set of 91 unique organic acids selected for clinical diagnostic purposes based on known metabolic diseases and their markers (Supplemental Table 1). Organic acids were reported in units of mmol/mol creatinine. Because the organic acid data were generated from a set of residual urine samples, the effect of sample age (storage time) on organic acid profiles was assessed as described below (see Results).
Analysis of VPA and Metabolites
Urinary VPA and metabolite concentrations were determined for the primary study according to published validated methods (38–40). Separate methods were employed for determination of CYP- and β-oxidation-generated metabolites (38) and for the thioether N-acetylcysteine (NAC) conjugates, 5-NAC-3-ene VPA (NAC I), 5-NAC-2-ene VPA (NAC II) and NAC total (39, 40).
Statistical Analysis
Of the 91 organic acids reported for each sample, 71 were included in the statistical analysis (Table 2). The 20 excluded acids showed no detectable levels in any of the urine samples, thus contributing no variation to the data set. Additionally, results for some analytes were reported as 0 mmol/mol creatinine for several subjects. To facilitate logarithmic transformation and statistical analysis, undetectable concentrations were arbitrarily assigned a value ten times smaller than the minimum reported value for those analytes. One VPA subject (a 4 year old male) was classified as an outlier, with 36 acids outside the upper quartile + 1.5(interquartile range) − up to 15 times outside the next nearest value. This subject was excluded from data modeling.
Statistical analyses were performed using JMP Statistical Software, v. 8.0 (SAS, Cary, NC). Principal components analysis (PCA) was performed on the data correlation matrix to uncover the major sources of variation in the dataset. The first principal component (PC1) represents a linear combination of the most important variables that account for as much of the variability in the data as possible. PC2 and each succeeding component accounts for highest variance possible under the constraint that it be orthogonal (unrelated) to the preceding components. The first two principal components were regressed against demographic variables such as primary group, age, weight, dose (for VPA and CBZ subjects), gender and race. The alpha level was set at 0.05. A linear discriminant model was also calculated using “group” (VPA/CBZ/Control) as the classifying variable and the 71 organic acids as the covariates.
Supplementary Material
Acknowledgments
This research was supported by grant U01 HD044239 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development using resources provided by the NICHD Network of Pediatric Pharmacology Research Units (PPRU) at Children’s Mercy Hospitals and Clinics, Kansas City, MO (5 U10 HD031313), Primary Children’s Medical Center/University of Utah, Salt Lake City, UT (5 U10 HD045986) and Kosair Children’s Hospital, Louisville, KY (5 U10 HD045934). The authors acknowledge the assistance of T.K.L. Kiang and X.W. Teng (UBC) with valproic acid and metabolite analysis.
Abbreviations
- CBZ
carbamazepine
- CYP
cytochrome P450
- PCA
principal components analysis
- VPA
valproic acid
- GC/MS
gas chromatography/mass spectrometry
Footnotes
Supplementary information is available at http://www.nature.com/cpt
Conflict of Interest
The authors declare no conflicts of interest.
References
- 1.Bryant AE, Dreifuss FE. Valproic acid hepatic fatalities. III U.S experience since 1986. Neurology. 1996;46:465–469. doi: 10.1212/wnl.46.2.465. [DOI] [PubMed] [Google Scholar]
- 2.Dreifuss FE, Langer DH, Moline KA, Maxwell JE. Valproic acid hepatic fatalities. II US experience since 1984. Neurology. 1989;39:201–207. doi: 10.1212/wnl.39.2.201. [DOI] [PubMed] [Google Scholar]
- 3.Dreifuss FE, Santilli RN, Langer DH, Sweeney KP, Moline KA, Menander KB. Valproic acid hepatic fatalaties: a retrospective review. Neurology. 1987;37:379–385. doi: 10.1212/wnl.37.3.379. [DOI] [PubMed] [Google Scholar]
- 4.Bjorge S, Baillie TA. Studies on the β-oxidation of valproic acid in rat liver mitochondrial preparations. Drug Metab Disp. 1991;19:823–829. [PubMed] [Google Scholar]
- 5.Li J, Norwood DL, Mao LF, Schulz H. Mitochondrial metabolism of valproic acid. Biochemistry. 1991;30:388–394. doi: 10.1021/bi00216a012. [DOI] [PubMed] [Google Scholar]
- 6.Lheureux PER, Hantson P. Carnitine in the treatment of valproic acid-induced toxicity. Clin Toxicol. 2009;47:101–111. doi: 10.1080/15563650902752376. [DOI] [PubMed] [Google Scholar]
- 7.Aires CCP, Ruiter JPN, Luis PBM, ten Brink HJ, Ijlst L, de Almeida IT, et al. Studies on the extra-mitochondrial CoA-ester formation of valproic and Δ4-valproic acids. Biochim Biophys Acta. 2007;1771:533–543. doi: 10.1016/j.bbalip.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Kassahun K, Hu P, Grillo MP, Davis MR, Jin L, Baillie TA. Metabolic activation of unsaturated derivatives of valproic acid. Identification of novel glutathione adducts formed through coenzyme A-dependent and -independent processes. Chem-Biol Interact. 1994;90:253–275. doi: 10.1016/0009-2797(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 9.Tong V, Teng XW, Chang TKH, Abbott FS. Valproic acid I: time course of lipid peroxidation biomarkers, liver toxicity, and valproic acid metabolite levels in rats. Toxicol Sci. 2005;86:427–435. doi: 10.1093/toxsci/kfi184. [DOI] [PubMed] [Google Scholar]
- 10.Tong V, Teng XW, Chang TKH, Abbott FS. Valproic acid II: Effects on oxidative stress, mitochondrial membrane potential, and cytotoxicity in glutathione-depleted rat hepatocytes. Toxicol Sci. 2005;86:436–443. doi: 10.1093/toxsci/kfi185. [DOI] [PubMed] [Google Scholar]
- 11.Silva MFB, Ruiter JPN, Overmars H, Bootsma AH, van Gennip AH, Jakobs C, et al. Complete β-oxidation of valproate: cleave of 3-oxovalproyl-CoA by a mitochondrial 3-oxoacyl-CoA thiolase. Biochem J. 2002;362:755–760. doi: 10.1042/0264-6021:3620755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee MS, Jung BH, Chung BC, Cho SH, Kim KY, Kwon OS, et al. Metabolomics study with gas chromatoraph-mass spectrometry for predicting valproic acid-induced hepatotoxicity and discovery of novel biomarkers in rat urine. Int J Toxicol. 2009;28:392–404. doi: 10.1177/1091581809340329. [DOI] [PubMed] [Google Scholar]
- 13.Beger RD, Hansen DK, Schnackenberg LK, Cross BM, Fatollahi JJ, Lagunero FT, et al. Single valproic acid treatment inhibitis glycogen and RNA ribose turnover while disrupting glucose-derived cholesterol synthesis in liver as revealed by the [U-13C6]-D-glucose tracer in mice. Metabolomics. 2009;5:336–345. doi: 10.1007/s11306-009-0159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schnackenberg LK, Jones RC, Thyparambil S, Taylor JT, Han T, Tong W, et al. An integrated study of acute effects of valproic acid in the liver using metabonomics, proteomics, and transcriptomics platforms. OMICS. 2006;10:1–14. doi: 10.1089/omi.2006.10.1. [DOI] [PubMed] [Google Scholar]
- 15.Aires CCP, Soveral G, Luis PBM, ten Brink HJ, de Almeida IT, Duran M, et al. Pyruvate uptake is inhibited by valproic acid and metabolites in mitochondrial membranes. FEBS Lett. 2008;582:3359–3366. doi: 10.1016/j.febslet.2008.08.028. [DOI] [PubMed] [Google Scholar]
- 16.Silva MFB, Ruiter JPN, Jakobs C, Duran M, de Almeida IT, Wanders RJA. Valproate inhibits the mitochondrial pyruvate-driven oxidative phosphorylation in vitro. J Inherit Metab Dis. 1997;20:397–400. doi: 10.1023/a:1005398516208. [DOI] [PubMed] [Google Scholar]
- 17.Verrotti A, Manco R, Agostinelli S, Coppola G, Chiarelli F. The metabolic syndrome in overweight epileptic patients treated with valproic acid. Epilepsia. 2009;51:268–273. doi: 10.1111/j.1528-1167.2009.02206.x. [DOI] [PubMed] [Google Scholar]
- 18.Novak GP, Maytal J, Alshansky A, Eviatar L, Sy-Kho R, Siddique Q. Risk of excessive weight gain in epileptic children treated with valproate. J Child Neurol. 1999;14:490–495. doi: 10.1177/088307389901400802. [DOI] [PubMed] [Google Scholar]
- 19.Kochen W, Schneider A, Ritz A. Abnormal metabolism of valproic acid in fatal hepatic failure. Eur J Pediatr. 1983;141:30–35. doi: 10.1007/BF00445664. [DOI] [PubMed] [Google Scholar]
- 20.Tennison MB, Miles MV, Pollack GM, Thorn MD, Dupuis RE. Valproate metabolites and hepatotoxicity in an epileptic population. Epilepsia. 1988;29:543–547. doi: 10.1111/j.1528-1157.1988.tb03758.x. [DOI] [PubMed] [Google Scholar]
- 21.Kuhara T, Inoue Y, Matsumoto M, Shinka T, Matsumoto I, Kawahara N, et al. Markedly increased ω-oxidation of valproate in fulminant hepatic failure. Epilepsia. 1990;31:214–217. doi: 10.1111/j.1528-1167.1990.tb06309.x. [DOI] [PubMed] [Google Scholar]
- 22.Fisher E, Siemes H, Pund R, Wittfohy W, Nau H. Valproate metabolites in serum and urine during antiepileptic therapy in children with infantile spasms: Abnormal metabolite pattern associated with reversible hepatotoxicity. Epilepsia. 1992;33:165–171. doi: 10.1111/j.1528-1157.1992.tb02301.x. [DOI] [PubMed] [Google Scholar]
- 23.Kondo T, Kaneko S, Otani K, Ishida M, Hirano T, Fukushima Y, et al. Associations between risk factors for valproate hepatoxicity and altered valproate metabolism. Epilepsia. 1992;33:172–177. doi: 10.1111/j.1528-1157.1992.tb02302.x. [DOI] [PubMed] [Google Scholar]
- 24.Siemes H, Nau H, Schultze K, Wittfoht W, Drews E, Penzien J, et al. Valproate (VPA) metabolites in various clinical conditions of probable VPA-associated hepatotoxicity. Epilepsia. 1993;34:332–346. doi: 10.1111/j.1528-1157.1993.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 25.Rettie AE, Rettenmeier AW, Howald WN, Baillie TA. Cytochrome P-450-catalyzed formation of Δ4-VPA, a toxic metabolite of valproic acid. Science. 1987;235:890–893. doi: 10.1126/science.3101178. [DOI] [PubMed] [Google Scholar]
- 26.Gopaul SV, Farrell K, Abbott FS. Effects of age and polytherapy, risk factors of valproic acid (VPA) hepatotoxicity, on the excretion of thiol conjugates of (E)-2,3-diene VPA in people with epilepsy taking VPA. Epilepsia. 2003;44:322–328. doi: 10.1046/j.1528-1157.2003.07202.x. [DOI] [PubMed] [Google Scholar]
- 27.Boulat O, Gradwohl M, Matos V, Guignard JP, Bachmann C. Organic acids in the second morning urine in a healthy swiss paediatric population. Clin Chem Lab Med. 2003;41:1642–1658. doi: 10.1515/CCLM.2003.248. [DOI] [PubMed] [Google Scholar]
- 28.Guneral F, Bachmann C. Age-related reference values for urinary organic acids in a healthy Turkish pediatric population. Clin Chem. 1994;40:862–868. [PubMed] [Google Scholar]
- 29.Thompson JA, Miles BS, Fennessey PV. Urinary organic acids quantitated by age groups in a healthy pediatric population. Clin Chem. 1977;23:1734–1738. [PubMed] [Google Scholar]
- 30.Kumps A, Duez P, Mardens Y. Metabolic, nutritional, iatrogenic, and artifactual sources of urinary organic acids: a comprehensive table. Clin Chem. 2002;48:708–717. [PubMed] [Google Scholar]
- 31.Finney H, Newman DJ, Thakkar H, Fell JME, Price CP. Reference ranges for plasma cystatin C and creatinine measurements in premature infants, neonates, and older children. Arch Dis Child. 2000;82:71–75. doi: 10.1136/adc.82.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Unay B, Akin R, Sarici SU, Gok F, Kurt I, Gokcay E. Evaluation of renal tubular function in children taking anti-epileptic treatment. Nephrology. 2006;11:485–488. doi: 10.1111/j.1440-1797.2006.00699.x. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz GJ. Does kL/PCr estimate GFR, or dose GFR determine k? Pediatr Nephrol. 1992;6:512–515. doi: 10.1007/BF00866487. [DOI] [PubMed] [Google Scholar]
- 34.Clayton TA, Lindon JC, Cloarec O, Antti H, Charuel C, Hanton G, et al. Pharmaco-metabonomic phenotyping and personalized drug treatment. Nature. 2006;440:1073–1077. doi: 10.1038/nature04648. [DOI] [PubMed] [Google Scholar]
- 35.Winnike JH, Li Z, Wright FA, Macdonald JM, O’Connell TM, Watkins PB. Use of Pharmaco-metabonomics for early prediction of acetaminophen-induced hepatotoxicity in humans. Clin Pharmacol Ther. 2010;88:45–51. doi: 10.1038/clpt.2009.240. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka K, West-Dull A, Hine DG, Lynn TB, Lowe T. Gas-chromatographic method of analysis for urinary organic acids. II Description of the procedure, and its application to diagnosis of patients with organic acidurias. Clin Chem. 1980;26:1847–1853. [PubMed] [Google Scholar]
- 37.Sweetman L. Organic acid analysis. In: Hommes FI, editor. Techniques in Diagnostic Human Biochemical Genetics: A Laboratory Manual. New York: Wiley-Liss; 1991. pp. 143–176. [Google Scholar]
- 38.Anari MR, Burton RW, Gopaul SV, Abbott FS. Metabolic profiling of valproic acid by cDNA-expressed human cytochrome P450 enzymes using negative-ion chemical ionization gas chromatography-mass spectrometry. J Chromatogr B. 2000;742:217–227. doi: 10.1016/s0378-4347(00)00161-4. [DOI] [PubMed] [Google Scholar]
- 39.Gopaul SV, Farrell K, Abbott FS. Identification and characterization of N-acetylcysteine conjugates of valproic acid in humans and animals. Drug Metab Disp. 2000;28:823–832. [PubMed] [Google Scholar]
- 40.Gopaul SV, Farrell K, Abbott FS. Gas chromatography/negative ion chemical ionization mass spectrometry and liquid chromatography/electrospray ionization tandem mass spectrometry quantitative profiling of N-acetylcysteine conjugates of valproic acid in urine: application in drug metabolism studies in humans. J Mass Spectrom. 2000;35:698–704. doi: 10.1002/1096-9888(200006)35:6<698::AID-JMS996>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.