Fig 1.
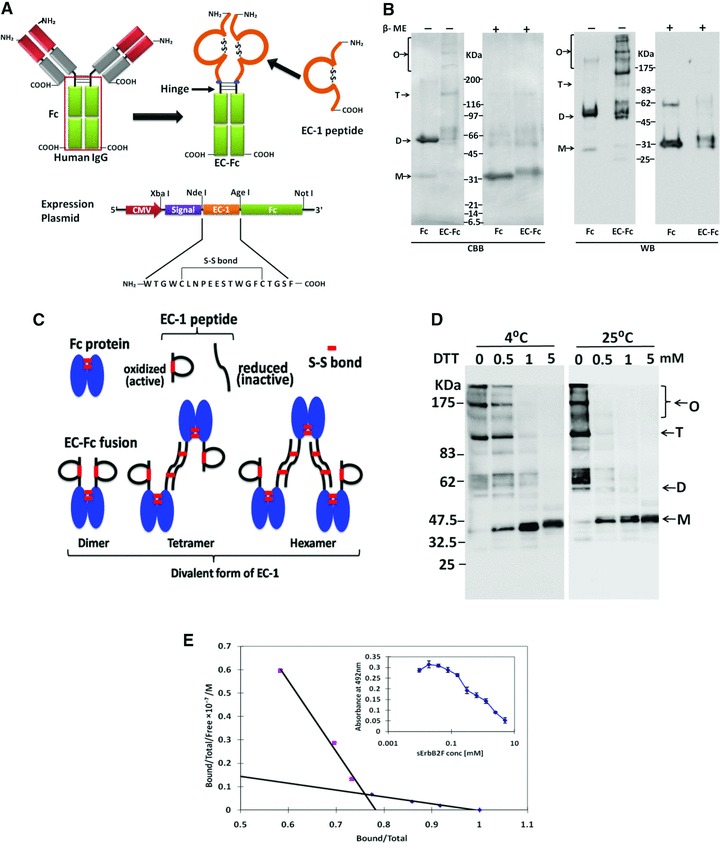
Construction and preparation of EC-Fc. (A) Schematic diagrams of EC-Fc. EC-1 peptide was fused to the human IgG Fc domain containing hinge region, so that the fusion protein EC-Fc should form dimer. The secretional expression of EC-Fc was designed with the signal peptide derived from human RNase I under the control of CMV promoter. The unique restriction enzyme sites Xba I, Nde I, Age I and Not I were used to construct the expression plasmid. (B) EC-Fc (theoretical MW = 30.0 kDa) or IgG Fc (theoretical MW = 27.6 kDa) was produced, purified with protein A-column and subjected to SDS-PAGE and Western blotting. Anti-human IgG Fc antibody recognized the purified EC-Fc without significant degradation. β-ME: betamercaptoethanol; CBB: Coomassie Brilliant Blue staining; WB: Western blotting. (C) Reduction of EC-Fc oligomeric form with DTT treatment. WB: Western blotting; M: monomer; D: dimer; T: tetramer; O: oligomer (B, C). Molecular weight markers range from 6.5 to 200 kDa (B, C). (D) Schematic representation of the probable dimer, tetramer and hexamer formation of EC-Fc and dimer formation of Fc through disulphide linkages. (E) Evaluation of the affinity of EC-Fc for ErbB2 by competitive EIA. Kd values between EC-Fc and sErbB2F were estimated from the Scatchard plots.
