Fig 1.
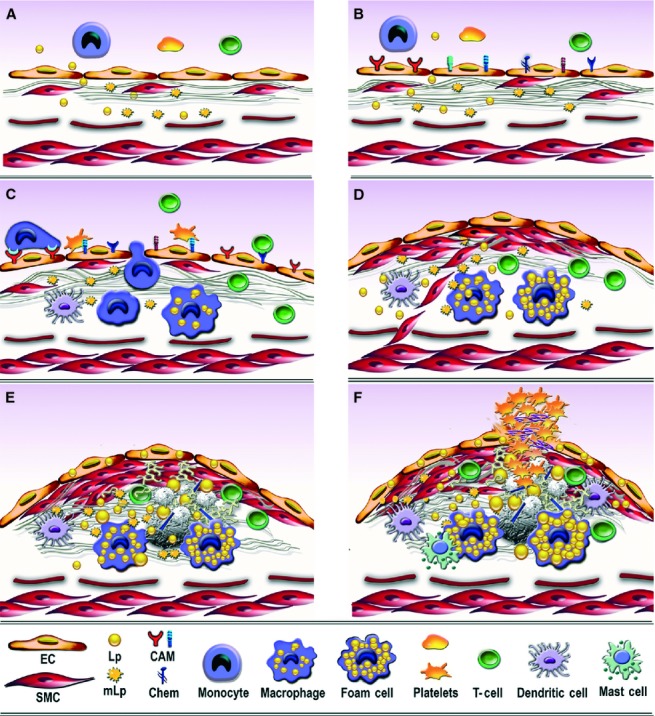
Consecutive arbitrary stages occurring in the development of atherosclerotic lesion in arterial lesion-prone areas. (A) Stage I. Endothelial cell activation/modulation of constitutive functions. The initial stage in atheroma formation in dyslipidaemia consists in endothelial cell (EC) increased transcytosis of plasma lipoproteins (Lp) and their housing in the subendothelium and a switch of the cells to a secretory phenotype responsible for the development of a hyperplasic basal lamina. Within the subendothelium, Lp, especially LDL by interaction with extracellular matrix components, changes its attributes becoming the highly atherogenic, oxidized modified lipoproteins (mLp). (B) Stage II. EC dysfunction. Affected on both sides, luminal by alterations of plasma homoeostasis and abluminal by the accrual of mLp, the EC initiate an inflammatory process manifested by the expression of new or more cell adhesion molecules, cytokines and chemokines (CAM and Chem), an indication of endothelial dysfunction. (C) Stage III. Recruitment of blood immune cells and commencement of a robust inflammatory reaction. Blood monocytes and T cells adhere to activated/dysfunctional EC and undergo directed diapedesis into the intima. Adhered platelets assist leucocytes migration. Within the intima, monocytes become activated macrophages that express scavenger receptors, which function in the uptake of mLp and the formation of foam cells that secrete a variety of proinflammatory mediators. Lymphocytes switch to activated pro-inflammatory (Th 1) and anti-inflammatory (Th 2 and TREG) cells that secrete cytokines and chemokines. The direct or indirect crosstalk between resident and migrated cells within the intima dictates the lesion progression. Activated dendritic cells contribute to T cells recruitment and activation within the plaque. (D) Stage IV. SMC-key participants to fibrous plaque formation. The proliferation of intima-resident SMC and of SMC migrated from the media to the intima leads to the formation of a fibrous cap that is accompanied by increased synthesis of extracellular matrix components. (E) Stage V. Resident and immune cells and the factors they secrete generate a calcified fibro-lipid plaque. SMC-, macrophages-derived foam cells, apoptotic cells-derived lipids and calcification centres form a lipid loaded necrotic core rich in cholesterol crystals. (F) Stage VI. The unstable fibro-lipid plaque: rupture and thrombosis EC apoptosis and erosion, thinning of the fibrous cap, cell apoptosis, macrophages, dendritic and mast cells-secreted pro-inflammatory mediators generate the physical rupture of the plaque. This ends in direct contact between tissue factors and circulating platelets and blood coagulation components triggering the thrombus formation that may partially or totally imped the blood flow leading to either myocardial infarction or stroke.
