Abstract
The prognosis of metastatic cancer patients is still largely affected by treatment failure, mainly due to drug resistance. The hypothesis that chemotherapy might miss circulating tumour cells (CTCs) and particularly a subpopulation of more aggressive, stem-like CTCs, characterized by multidrug resistance, has been recently raised. We investigated the prognostic value of drug resistance and stemness markers in CTCs from metastatic colorectal cancer patients treated with oxaliplatin (L-OHP) and 5-fluoruracil (5-FU) based regimens. Forty patients with metastatic colorectal cancer were enrolled. CTCs were isolated from peripheral blood and analysed for the expression of aldheyde dehydrogenase 1 (ALDH1), CD44, CD133 (used as markers of stemness), multidrug resistance related protein 5 (MRP5 used as marker of resistance to 5-FU and L-OHP) and survivin (used as a marker of apoptosis resistance). CTCs were found in 27/40 (67%) patients. No correlation was found between the expression of either CD44 and CD133 in CTCs and the outcome of patients, while a statistically significant shorter progression-free survival was found in patients with CTCs positive for the expression of ALDH1, survivin and MRP5. These results support the idea that isolating survivin and MRP5+ CTCs may help in the selection of metastatic colorectal cancer patients resistant to standard 5-FU and L-OHP based chemotherapy, for which alternative regimens may be appropriate.
Keywords: colorectal cancer, circulating tumour cells, drug resistance, aldheyde dehydrogenase 1 survivin, multidrug resistance related protein 5
Introduction
Colorectal cancer represents the second leading cause of cancer-related deaths due to therapy resistance both in Europe and United States. Drug resistance is thought to cause treatment failure in over 90% of patients with metastatic cancer, while drug resistant micrometastic tumour cells may also reduce the impact of adjuvant chemotherapy treatment [1].
Drug resistance, underlying tumour recurrence and the lack of curative treatments in metastatic disease, raises the question whether conventional anticancer therapies target the right cells. Indeed, these treatments might miss circulating tumour cells (CTCs), and particularly the chemoresistant and radioresistant subpopulation within them.
Recent evidences suggest that the count of CTCs has prognostic significance in metastatic colorectal cancer, as well as in other epithelial malignancies. It has been suggested that more aggressive CTCs share genotypic characteristics with cancer stem cells, characterized by multidrug resistance, which allow these cells to drive tumour growth evading apoptosis and conventional therapy [2]. In colorectal cancer, specifically, CD44, CD133 and aldheyde dehydrogenase 1 (ALDH1) have been recently suggested as potential markers of colorectal cancer stemness [3]. Apoptosis evasion through the overexpression of survivin also represents one of the mechanisms by which tumour cells acquire the ability to enter into the blood flow and escape to drug-induced death. In colon cancer, specifically, the induction of survivin signalling pathway represents one mechanism of resistance to oxaliplatin (L-OHP) and 5-fluoruracil (5-FU) by modulating caspase-dependent and caspase-independent apoptosis [4]. Furthermore, the expression of multidrug resistance related protein 5 (MRP5), belonging to ATP binding cassette transporters family, is associated to resistance to 5-FU and platin compounds [5]. In a previous study, we demonstrated that a drug resistance profile on CTCs from epithelial cancers through the evaluation of specific MRPs is predictive of resistance to chemotherapy, independently of tumour type and stage of disease [6, 7].
We thus investigated the prognostic significance of drug resistance and stemness markers in CTCs isolated from metastatic colorectal cancer patients treated with L-OHP and 5-FU based regimens.
Patients and methods
Forty (40) patients with metastatic colorectal cancer were enrolled between October 2007 and October 2009 at ‘Sapienza’ University of Rome. Inclusion criteria were measurable metastatic colorectal cancer, commencement of a new systemic therapy with 5-FU and L-OHP, with Eastern Cooperative Oncology Group performance status score of 0 to 2. All patients signed an informed consent. CTCs were isolated from 10 cc of peripheral blood by CELLection™ Dynabeads® coated with the monoclonal antibody towards the human epithelial cell adhesion molecule. From the isolated CTCs cDNAs were synthesised and subjected to PCR amplifications specific for CEA (used as a marker for colorectal cancer cells) ALDH1, CD44, CD133 (used as markers of stemness), MRP5 (used as marker of resistance to 5-FU and L-OHP) and survivin (used as a marker of apoptosis resistance). Statistical analysis was performed with BMDP statistical software, version 7 (Statistical Solutions, Saugus, MA, USA) and SPSS (Chicago, version 15.00 for Windows). Progression-free survival (PFS) was defined as the time elapsed between the date of blood sampling, corresponding to the start of treatment and the date of clinical disease progression or death for any cause.
Kaplan–Meier product-limit method was used to correlate PFS with expression of survivin, MRP5, ALDH1, CD133 and CD44. Different prognostic groups were compared using the log rank test. A P-value of less than .05 was considered statistically significant.
Results
Twenty-seven of 40 (67%) patients were found positive for the presence of CTCs. In the group of CTC+ patients, 12/27 (45%) had progression of disease (PD) and 15/27 (55%) had a clinical response (CR) during the time course of chemotherapy with L-OHP and 5-FU based regimens.
An amplification reaction with CEA primers was performed on RNAs extracted from CTC+ patients; all 27 samples were found positive.
ALDH1+ CTCs were detected in 9 (33%) out of 27 CTC+ patients. Among these ALDH1+ patients, six had a PD and three a CR. A significant correlation between ALDH1 expression and poor outcome (P∇ 0.046) was found (Fig. 1A). CD44+ and CD133+ CTCs were found in 3/27 (11%) and 2/27 (7%) patients, respectively. No correlation was found between expression of either CD44 and CD133 or outcome of patients (P∇ 0.8 for both). Survivin was detected in CTCs from 13/27 (48%) patients. In the group of survivin positive patients, 10/13 (77%) had PD and 3/13 (23%) had CR. In the group of 14 survivin negative patients, PD was observed in 2/14 (14%) of cases, and CR in 12/14 (86%). The difference in PFS between the two groups was found statistically significant (P < 0.001) (Fig. 1B). MRP5, which transports L-OHP and 5-FU, was found expressed in CTCs from 15/27 patients (55%). Among these patients, 12 were in PD and 3 in CR. MRP5+ patients had a significantly shorter PFS compared to patients whose CTCs were negative for MRP5 expression (P < 0.001) (Fig. 1C). In Fig. 2, RT-PCR amplification products for all genes examined in CTCs from four exemplificative patients are shown. The expression of all markers analysed on CTCs in the population with and without drug resistance is shown in Table 1.
Fig 1.
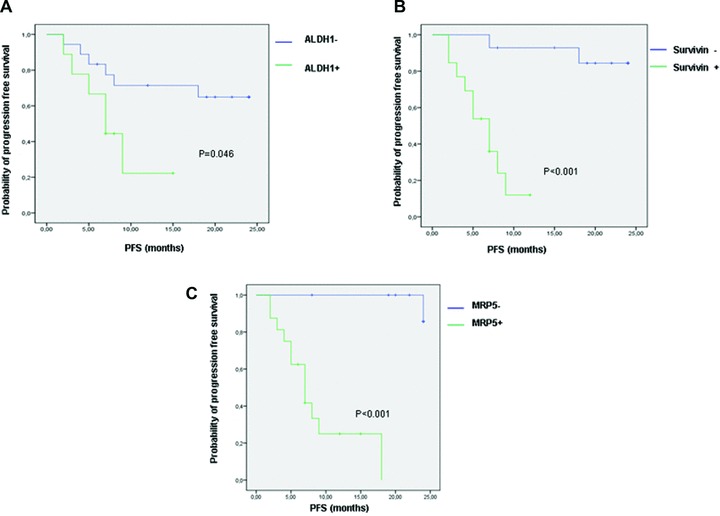
Kaplan–Meier curves showing the difference in PFS between patients with CTCs positive and negative for ALDH1 (A), survivin (B) and MRP5 (C) expression.
Fig 2.
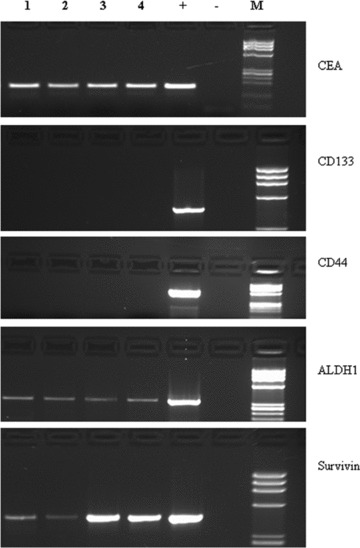
CEA, CD133, CD44, ALDH-1, MRP5 and survivin RT-PCR amplification products obtained by CTCs isolated from four colorectal cancer patients (lanes 1–4) loaded on 2% agarose gel; +, positive control; –, negative control (sample without RNA); M, molecular weight marker.
Table 1.
The expression of CD44, CD133, ALDH1, survivin and MRP5 in a population with and without resistance to chemotherapy is shown. In the same population response to therapy (PD = progressive disease; CR = clinical response) and PFS in months are indicated.
| Pts | CD44 | CD133 | ALDH1 | Survivin | MRP5 | Response to therapy | PFS |
|---|---|---|---|---|---|---|---|
| 1 | Neg | Neg | Neg | Neg | Neg | CR | 24 |
| 2 | Neg | Neg | Neg | Neg | Neg | CR | 24 |
| 3 | Neg | Neg | Neg | Neg | Neg | CR | 24 |
| 4 | Neg | Neg | Neg | Pos | Pos | CR | 6 |
| 5 | Neg | Neg | Neg | Pos | Pos | PD | 8 |
| 6 | Neg | Neg | Neg | Pos | Pos | CR | 12 |
| 7 | Neg | Neg | Neg | Neg | Neg | CR | 24 |
| 8 | Neg | Neg | Neg | Pos | Pos | PD | 2 |
| 9 | Neg | Neg | Neg | Neg | Neg | CR | 24 |
| 10 | Neg | Neg | Neg | Pos | Pos | PD | 5 |
| 11 | Neg | Neg | Pos | Pos | Pos | PD | 3 |
| 12 | Neg | Neg | Neg | Neg | Neg | CR | 19 |
| 13 | Neg | Neg | Pos | Pos | Pos | PD | 9 |
| 14 | Neg | Neg | Pos | Neg | Pos | PD | 7 |
| 15 | Neg | Neg | Pos | Neg | Neg | CR | 15 |
| 16 | Neg | Pos | Pos | Pos | Pos | PD | 7 |
| 17 | Neg | Neg | Neg | Pos | Pos | PD | 7 |
| 18 | Neg | Neg | Pos | Pos | Pos | PD | 5 |
| 19 | Neg | Neg | Neg | Neg | Neg | CR | 24 |
| 20 | Pos | Neg | Pos | Pos | Pos | PD | 2 |
| 21 | Neg | Neg | Pos | Pos | Pos | CR | 7 |
| 22 | Neg | Neg | Neg | Neg | Neg | CR | 22 |
| 23 | Neg | Neg | Neg | Pos | Pos | PD | 4 |
| 24 | Pos | Neg | Neg | Neg | Neg | CR | 20 |
| 25 | Neg | Neg | Neg | Neg | Pos | PD | 18 |
| 26 | Neg | Neg | Neg | Neg | Neg | CR | 24 |
| 27 | Pos | Pos | Pos | Neg | Neg | CR | 8 |
Discussion
Recent in vitro studies suggest that survivin expression represents a significant and independent predictor of resistance to L-OHP and 5-FU. Consistently with this observation, we describe for the first time that expression of survivin is a significant indicator of poor response to 5-FU and L-OHP in CTCs from metastatic colorectal cancer patients. Thus, the first conclusion is that the detection of survivin-positive CTCs may be predictive of poor response to chemotherapy in metastatic colorectal cancer. In a similar manner, patients whose CTCs express MRP5 had a shorter PFS and higher progression rate when treated with 5-FU and L-OHP specific MRP5 substrates. Preliminary data obtained in vitro have suggested that survivin plays a role in the chemoresistance mediated by p-glicoprotein (Pgp) in breast cancer cells by modulating the turnover of Pgp or transport by Pgp in the cell, which then results in anti-apoptosis and drug resistance [8]. Furthermore, the association between survivin and Pgp has been correlated to poor prognosis of patients [9].
In our series a similar concomitant expression of survivin and MRP5 was found in CTCs from 13/15 (87%) patients, all characterized by early progression of disease; we thus suggest that the expression of survivin may directly interfere with MRP5 mediated drug resistance, resulting in chemotherapy failure.
In addition, it has been demonstrated that drug resistance is often associated with ALDH1 expression in several cancer tissues; thus it was expected that ALDH1 would be associated with poor prognosis due to chemotherapy inefficiency. Corresponding experimental results for CTCs are still outstanding, except for breast cancer, where it has been recently demonstrated that the expression of ALDH1 among CTCs is associated with therapy failure [10]. To date, no similar data on CTCs have been reported for colorectal cancer.
In conclusion, these results may suggest that isolating survivin and MRP5 expressing CTCs may help in the selection of metastatic colorectal cancer patients resistant to 5-FU and L-OHP, for which alternative chemotherapy regimens may be appropriate. This addresses the important question whether CTCs can guide therapy choice and whether molecular analyses of these cells can aid in patient selection for targeted agents.
Acknowledgments
This work was partially supported by F.O.RO. (Fondazione Oncologica Romana).
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Negin BP, Cohen SJ. Circulating tumor cells in colorectal cancer: past, present, and future challenges. Curr Treat Options Oncol. 2010 doi: 10.1007/s11864-010-0115-3. DOI: 10.1007/s11864-010-0115-3. [DOI] [PubMed] [Google Scholar]
- 3.Huang EH, Wicha MS. Colon cancer stem cells: implications for prevention and therapy. Trends Mol Med. 2008;14:503–9. doi: 10.1016/j.molmed.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brenes O, Arce F, Gätjens-Boniche O, et al. Characterization of cell death events induced by anti-neoplastic drugs cisplatin, paclitaxel and 5-fluorouracil on human hepatoma cell lines: possible mechanisms of cell resistance. Biomed Pharmacother. 2007;61:347–55. doi: 10.1016/j.biopha.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Zhou SF, Wang LL, Di YM, et al. Substrates and inhibitors of human multidrug resistance associated proteins and the implications in drug development. Curr Med Chem. 2008;15:1981–2039. doi: 10.2174/092986708785132870. [DOI] [PubMed] [Google Scholar]
- 6.Gazzaniga P, Gradilone A, Naso G, et al. Chemoresistance profile of circulating tumor cells: toward a clinical benefit. Int J Cancer. 2008;123:1730–2. doi: 10.1002/ijc.23699. [DOI] [PubMed] [Google Scholar]
- 7.Gazzaniga P, Naso G, Gradilone A, et al. Chemosensitivity profile assay of circulating cancer cells: prognostic and predictive value in epithelial tumors. Int J Cancer. 2010;126:2437–47. doi: 10.1002/ijc.24953. [DOI] [PubMed] [Google Scholar]
- 8.Liu F, Xie ZH, Cai GP, et al. The effect of survivin on multidrug resistance mediated by P-glycoprotein in MCF-7 and its adriamycin resistant cells. Biol Pharm Bull. 2007;30:2279–83. doi: 10.1248/bpb.30.2279. [DOI] [PubMed] [Google Scholar]
- 9.Nakagawa Y, Abe S, Kurata M, et al. IAP family protein expression correlates with poor outcome of multiple myeloma patients in association with chemotherapy-induced overexpression of multidrug resistance genes. Am J Hematol. 2006;81:824–31. doi: 10.1002/ajh.20656. [DOI] [PubMed] [Google Scholar]
- 10.Theodoropoulos PA, Polioudaki H, Agelaki S, et al. Circulating tumor cells with a putative stem cell phenotype in peripheral blood of patients with breast cancer. Cancer Lett. 2010;288:99–106. doi: 10.1016/j.canlet.2009.06.027. [DOI] [PubMed] [Google Scholar]


