Figure 1.
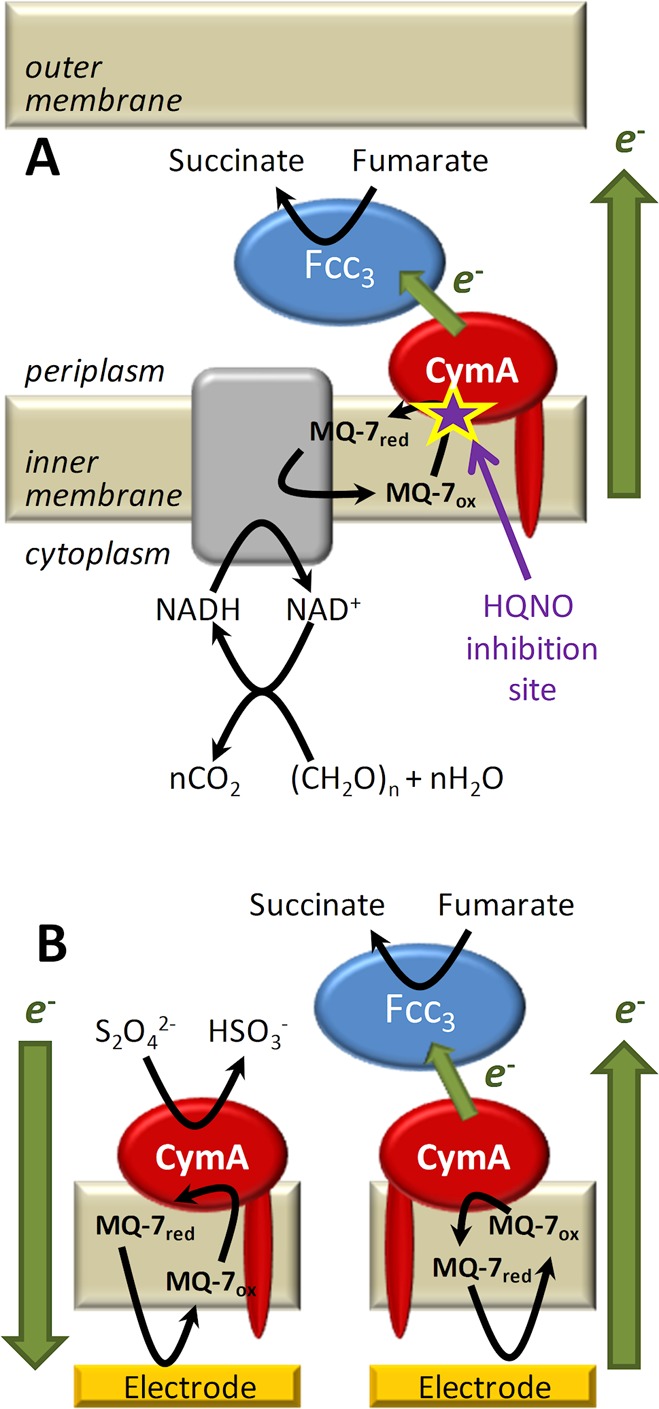
(A) Schematic of the inner membrane respiratory chain of Shewanella grown anaerobically with fumarate as the terminal electron acceptor. Electrons generated during catabolism are donated to the MQ-7 pool, which is reoxidized by CymA. Based on primary sequence analysis,46 homology to NrfH for which a crystal structure is available,38 and biochemical analysis of both CymA and other NapC/NirT superfamily members, CymA is known to contain a single N-terminal transmembrane α-helix with a single globular “head” domain facing the periplasm. CymA transfers the electrons to the periplasmic enzyme flavocytochrome c3 (Fcc3), which reduces fumarate to succinate. The site of action of the competitive inhibitor, 2-n-heptyl-4-hydroxyquinoline N-oxide (HQNO), is indicated in magenta. (B) Schematic of CymA containing inner-membrane architecture on an electrode surface in the presence of Fcc3 and a chemical reducing agent dithionite (S2O42–). In both panels, chemical reactions and ET steps are shown with black and green arrows, respectively.
