1.
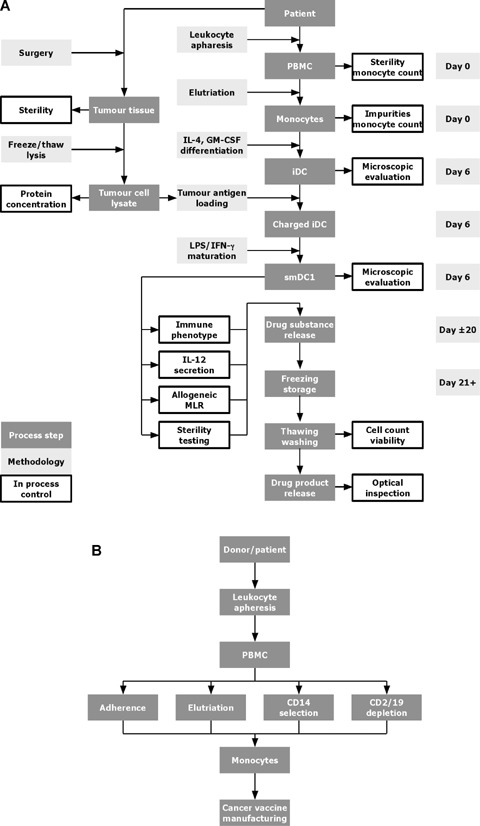
Dendritic cell (DC) manufacturing. (A) Flow chart of the standard operating procedure (SOP) for the manufacturing of a cancer vaccine. From patients undergoing tumour surgery a piece of tumour tissue is delivered to the manufacturing facility. The tumour tissue is disrupted mechanically, and the tumour cells are lysed to enrich soluble protein containing tumour antigens. After recovery from surgery, leucocyte apheresis is performed to collect peripheral blood mononuclear cells (PBMCs) from the patients, the monocytes are enriched and cultivated for 6 days in the presence of interleukin (IL)-4 and granulocyte-macrophage colony-stimulation factor (GM-CSF) in order to obtain iDCs. The iDCs are charged with tumour antigens, exposed to LPS/IFN-γ to trigger maturation and cryopreserved until treatment. An aliquot of the DC cancer vaccine is subjected to quality control, a potency assay and sterility control. If all criteria are met the DC cancer vaccine is released for treatment. (B) Flow chart of DC manufacturing using different monocyte enrichment protocols. Monocytes are isolated from PBMCs of a healthy donor or cancer patient using leucocyte apheresis. The PBMCs are further subjected to monocyte enrichment using adherence, or semi-automated elutriation, CD14 selection, or CD2/19 depletion. The differentiation into DCs is done in the same way independently of the enrichment procedure used (see Fig. 1A).
