Fig 3.
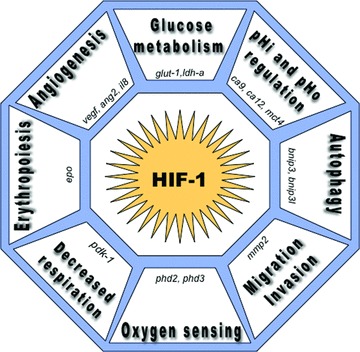
HIF-1 mediates microenvironmental changes and cellular adaptation to tumour hypoxia. After translocation to the nucleus, dimerization of HIF-1α with the constitutive HIF-1β, forms an active transcription factor that only exists in a low oxygen tension (hypoxia). Heterodimerization and DNA binding involve interaction between the N-terminal basic-helix-loop-helix (bHLH) and PerArntSim domains. HIF-1 binds hypoxia responsive elements (HRE) in target genes and activates the transcription of genes enhancing adaptation and cell survival in a hypoxic environment. These target genes play key roles in oxygen sensing via the prolyl hydroxylases 2 and 3 (phd2, phd3), in decreased mitochondrial respiration through the induction of pdk1, in erythropoiesis (epo), in angiogeneisis via the expression of vascular endothelial growth factor (vegf), angiopoietin 2 (ang2), interleukin 8 (il8), in increased glycolytic enzymes as for example glucose transporters (glut-1), lactate dehydrogenase-a (ldh-a), in pHi and pHo regulation with HIF-1 induction of the carbonic anhydrase 9 and 12 (ca9, ca12) and the mct4, in autophagy triggered by Bcl-2/adenovirus EIB 19-kD interacting protein 3 (bnip3) and bnip3l, and in migration/invasion mediated by HIF induction of matrix metalloproteinase 2 (mmp2).
