Fig 1.
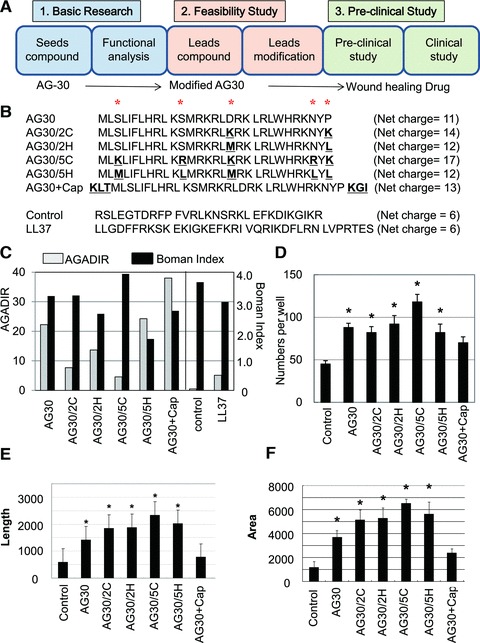
Several different modified AG30 peptides were created by replacing hydrophobic or cationic amino acids or adding capping structures. (A) Study design of this research. AG30 can be a good seed for wound-healing drug and this modified AG30 may correspond to be lead compounds as feasibility study toward clinical application (towards wound-healing drug). (B) Peptide sequences and net charge of modified AG30, control peptide and LL37. ‘*’ indicates positions where amino acids were replaced. ‘AG30/2C’ and ‘AG30/5C’ indicate the replacement of two or five cationic amino acid (from S, S, D, N, P to K, R, K, R, K, respectively). ‘AG30/2H’ and ‘AG30/5H’ indicate the replacement of two or five hydrophobic amino acids (from S, S, D, N, P to M, L, M, L, L, respectively). ‘AG30+Cap’ indicates the addition of a capping structure (Ac-KLT on the C-terminus and KGI-amide on the N-terminus). (C) In silico analysis of modified AG30. AGADIR (white bar) scores were calculated to predict the peptide structure and the Boman Index (black bar) was calculated to predict the pleiotropic effects of cationic peptides. (D) HAEC migration after treatment with modified AG30 for 24 hrs. (E, F) Tube formation after daily treatment with modified AG30 for 4 days; length and area were measured. N = 6 per group and duplicated. *P < 0.05 versus control, †P < 0.05 versus AG30.
