7.
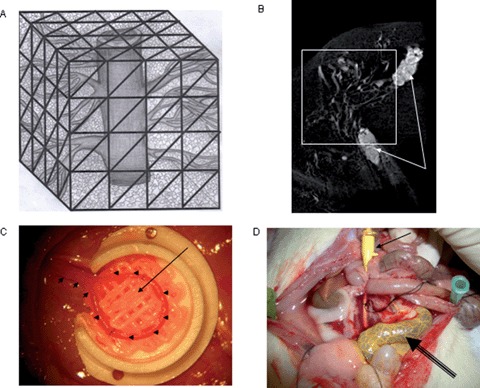
The majority of current tissue engineering approaches rely on the so called “extrinsic” mode of neovascularization. The neovascular bed originates from the periphery of the construct which should be implanted into a site of high vascularisation potential. Here recites a core limitation for transfer of tissue engineering models from the in vitro to the in vivo environment. Diffusion is the initial process involved, but it can only provide for cell nutrient supply and waste transport within a maximum range of 200 μm into the matrix. The survival of cells in the center of large cell containing constructs is therefore often limited by suboptimal initial vascularization. Cell labeling experiments have disclosed a considerable loss of osteoblasts within the first week following transplantation in porous cancellous bone matrices. Hence, reconstruction of small to moderate sized bone defects using extrinsic engineered bone tissues is technically feasible, and some of the currently developed concepts may represent alternatives to autologous bone grafts for certain clinical conditions, the reconstruction of large volume defects however remains challenging. Hence, reconstructive surgeons aim to generate so called “axially vascularized” tissues that can be transferred to the defect site using microsurgical techniques of vascular anastomosis. These tissues are immediately vascularized upon implantation into the defect as free flaps do Erol and Spira were the firast to report prefabrication of skin flaps by using an arteriovenous vessel which introduced this technique to the tissue engineering community. Most recently, the Horch/Kneser laboratory started collaboration with the Hutmacher laboratory to use the by AV-loop model (Figure C, small arrows) in combination with a composite scaffold (Figure C, long arrow shows with contrast agent filled loop, highly vascularized composite scaffold is found quadrant)) which is manufactured by a melt extrusion based SFF technique. Micro CT analysis (Figure B) of the with a construct agent perfused vascular network (Figure D, arrow show perfusion site with Microfil filled needle, double line arrow shows with Microfil filled vascular loop with caplillary network) of the engineered construct revealed a highly vascular zed construct.
