Abstract
Recent observational and clinical studies have raised interest in the potential health effects of cranberry consumption, an association that appears to be due to the phytochemical content of this fruit. The profile of cranberry bioactives is distinct from that of other berry fruit, being rich in A-type proanthocyanidins (PACs) in contrast to the B-type PACs present in most other fruit. Basic research has suggested a number of potential mechanisms of action of cranberry bioactives, although further molecular studies are necessary. Human studies on the health effects of cranberry products have focused principally on urinary tract and cardiovascular health, with some attention also directed to oral health and gastrointestinal epithelia. Evidence suggesting that cranberries may decrease the recurrence of urinary tract infections is important because a nutritional approach to this condition could lower the use of antibiotic treatment and the consequent development of resistance to these drugs. There is encouraging, but limited, evidence of a cardioprotective effect of cranberries mediated via actions on antioxidant capacity and lipoprotein profiles. The mixed outcomes from clinical studies with cranberry products could result from interventions testing a variety of products, often uncharacterized in their composition of bioactives, using different doses and regimens, as well as the absence of a biomarker for compliance to the protocol. Daily consumption of a variety of fruit is necessary to achieve a healthy dietary pattern, meet recommendations for micronutrient intake, and promote the intake of a diversity of phytochemicals. Berry fruit, including cranberries, represent a rich source of phenolic bioactives that may contribute to human health.
Introduction
The 2010 Dietary Guidelines for Americans recommends an increase in fruit intake as part of a healthy dietary pattern (1). These recommendations allow for a broad array of forms of fruit, including fresh, frozen, and canned, as well as dried fruit and fruit juices. The published guidelines provide as examples oranges and orange juice, apples and apple juice, bananas, grapes, raisins, and berries. Whereas berries are noted simply as good sources of potassium or fiber, recent research suggests that berry fruits are a rich source of numerous phytochemicals with a broad array of bioactivity and an impact on human health (2–6). Several berry fruit, including blackberries, blueberries, cranberries, raspberries, and strawberries, have recently received attention as a result of their effects in vitro and/or associations in observational studies with lowered risk of some chronic diseases. Randomized clinical trials have progressed sufficiently in recent years so that meta-analyses of these results have now been conducted.
Although not usually consumed raw, cranberry intake can be marked because of its presence in juices and sauces as well as its use as a dried fruit in cereal bars, cheeses, and chocolate and other confectionery. Also, cranberry powders and extracts are now used in foods products and dietary supplements. The American cranberry (Vaccinium macrocarpon) is a particularly rich source of (poly)phenols, which have been associated in vitro with antibacterial, antiviral, antimutagenic, anticarcinogenic, antitumorigenic, antiangiogenic, anti-inflammatory, and antioxidant properties (3, 7, 8). In vivo, animal models reveal that cranberry extracts can reduce C-reactive protein (CRP)11 and proinflammatory interleukins and increase NO synthesis (9); decrease angiotensin-converting enzyme, angiotensin II, and angiotensin II type 1 receptor (10); suppress Helicobacter pylori infection (11); and improve pancreatic β-cell glucose responsiveness and functional β-cell mass (12). Some of these actions may underlie the results from clinical studies showing that cranberry products can lower LDL cholesterol (LDL-C) and total cholesterol (13), increase HDL cholesterol (HDL-C) while lowering the oxidative modification of LDL-C (14), improve endothelial function (15, 16), lower glycemic responses (17), elevate plasma antioxidant capacity (18–20), modulate ulcerogenic gastric H. pylori colonization (21, 22), decrease cariogenic Streptococcus mutans and total bacterial counts in saliva (23), reduce biomarkers of metabolic syndrome (4, 24), and protect against urinary tract infections (UTIs) (5, 25).
We review here the phytochemical composition of cranberries and several other berry fruit, as well as evidence suggesting the potential of cranberries to promote urinary tract and cardiovascular health.
Cranberry Bioactive Composition and Content
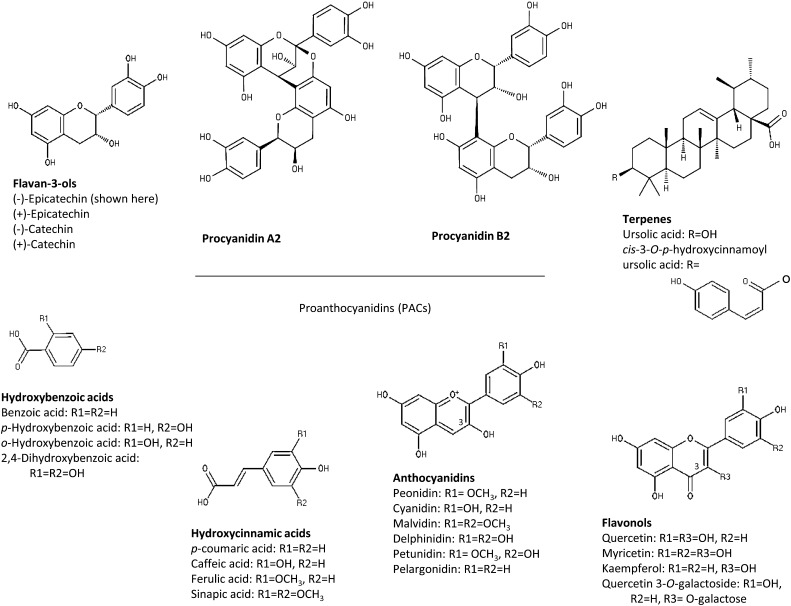
Proanthocyanidins.
American cranberry has a complex and rich phytochemical composition, particularly flavan-3-ols, A-type procyanidins (PACs), anthocyanins, benzoic acid, and ursolic acid. Cranberry flavan-3-ols are present as monomers, oligomers, and polymers (Table 1) (26). These oligomers and polymers are also referred to as PACs or condensed tannins and represent ∼85% of the total flavan-3-ols on a weight basis (27, 28). Cranberry PACs comprise a group of heterogeneous chemical structures, characterized by their constitutive units, types of linkage, and degree of polymerization (DP). (−)-Epicatechin is the predominant constitutive unit in cranberry PACs (Fig. 1), whereas (+)-catechin and (epi)gallocatechins are present only in trace amounts. The building blocks of PACs can be condensed either via a single C-C bond between C4 of the upper unit and C8 or C6 of the lower unit (B-type PACs) or with an additional ether-type bond between C2 of the upper unit and the hydroxyl group at C7 of the lower unit (A-type PACs) (Fig. 1). PACs with at least 1 A-type linkage account for 51–91% of total PACs in cranberry (29, 30). The distinction between A- and B-type PAC structures is of importance because the difference can influence their biological properties. The A-type PACs exhibit significantly greater inhibition of in vitro adhesion of P-fimbriated Escherichia coli bacteria to uroepithelial cells than the B-type PACs, the initial step of UTI (31). Many plant foods, such as apple, grape, and chocolate, contain high amounts of PACs, but only a few (plums, peanuts, avocados, cinnamon, lingonberry) contain A-type PACs, and none, except for lingonberry, at the amount found in cranberries (28, 32). Cranberries at 100 g fresh weight (FW) provide 419 ± 75 mg total flavan-3-ols, including 70 ± 13 mg oligomers with DP of 4–6, 63 ± 15 mg oligomers with DPs of 7–10, and 234 ± 49 mg polymers, whereas monomers, dimers, and trimers are present at lower amounts (7.3 ± 1.5, 26 ± 6, and 19 ± 3 mg, respectively) (33). These data are derived from 1 study in which a range of foods were analyzed for their PAC content (27). A few other quantitative data for cranberries have been published since this report, with estimates in the same range (27, 34). Importantly, analysis of the heterogeneous family of PACs, including numerous stereoisomers for which commercial standards are lacking, is still problematic, and data obtained with global methods do not address this issue. The average DP of PACs in cranberry has not been established. Foo et al. (29) initially reported an average DP of 4.7 for the cranberry PACs found to inhibit the in vitro adhesion of E. coli to uroepithelial cells. Subsequently, higher average DPs in cranberry PACs were found (8.5–15.3), and using matrix-assisted laser desorption-ionization time-of-flight MS, PACs with DPs as high as 23 were detected (26, 35).
TABLE 1.
Phytochemical content of cranberry foods
| Food source | Flavan-3-ol monomers and dimers (28, 49) | Proanthocyanidins (28, 34) | Anthocyanins (26, 34) | Hydroxybenzoic acids (49, 50) | Hydroxycinnamic acids (49, 50) | Terpenes (51) | Flavonols (50) |
| Cranberry fruit | |||||||
| mg/100 g | 7–33 | 133–367 | 13–171 | 503–602 | 73–82 | 65–125 | 20–40 |
| mg/serving1 | 5.6–26.4 | 106–293 | 10.4–136.8 | 402–482 | 57.6–65.6 | 52–100 | 16–32 |
| Cranberry juice | |||||||
| mg/L | 6–35 | 89–230 | 27–132 | 64 | 12–19 | Trace | 11–58 |
| mg/serving2 | 7 | 17.8–46 | 5.4–26.4 | 12.8 | 2.4–3.8 | Trace | 2.2–11.6 |
| Canned cranberry sauce | |||||||
| mg/100 g | 112.8 | 16–54.4 | 0.6–11.8 | 476 | 47.5 | 1.1–22.8 | —5 |
| mg/serving3 | 78.9 | 11.2–38 | 0.4–8.3 | 333.2 | 33.2 | 0.8–16 | — |
| Sweetened, dried cranberries | |||||||
| mg/100 g | — | 64.2 | 10.3 | — | — | 98.5 | — |
| mg/serving4 | — | 25.6 | 4.1 | — | — | 39.4 | — |
80 g whole fruit.
200 mL juice.
70 g sauce.
40 g dried fruit.
No data available.
FIGURE 1.
Cranberry bioactives. R in each structure indicates a point of variation within that class of bioactives, and these variations are defined underneath each structure.
Interestingly, cranberry contains a particularly high content of cell wall–bound PACs that are resistant to conventional methods of extraction (36). Thus, it is probable that the quantification of cranberry PACs in earlier literature is underestimated. The bound PACs should be considered as relevant to health outcomes because they have been shown to be bioaccessible in the human large intestine (37, 38).
Considering the limited data available today for the PAC content of cranberries, it is difficult to draw a reliable comparison to other berries. However, it is interesting to note that the qualitative profiles differ substantially. Jungfer et al. (32) found that 3 A-type trimers and procyanidin A2, identified as putative active compounds in V. macrocarpon, are present only in trace amounts in the European cranberry (Vaccinium oxycoccus L.), and at substantially higher amounts in lingonberry (Vaccinium vitis-idaea L.). Other differences were found in the flavan-3-ol profile of the 3 Vaccinium species, such as a much higher epicatechin:catechin ratio in American cranberry compared with the other berries. As in red wine, cranberry anthocyanins and proanthocyanins can be condensed in complex polymeric pigments at the last ripening stages or during postharvest storage. These structures have only begun to be characterized (35, 39).
Anthocyanins.
Amounts of anthocyanins are remarkably high in cranberry, contributing to the color of the fruit and derived foodstuffs, as well as the potential effects on human health. American cranberry is one of the rare foods that comprise glycosides of the 6 aglycones of the anthocyanidin family: cyanidin, peonidin, malvidin, pelargonidin, delphinidin, and petunidin (Fig. 1) (40). The predominant anthocyanins are the 3-O-galactosides and 3-O-arabinosides of cyanidin and peonidin; a total of 13 anthocyanins, mainly 3-O-monoglycosides, have been detected (26, 41). Cranberry anthocyanin content increases with ripening and is also dependent on the cultivar and size of the fruit (42–44). Pappas and Schaich (26) reported anthocyanin contents ranging from 13.6 to 171 mg/100 g FW. Anthocyanin profiles vary among berry species. Cranberry was reported as the main source of peonidin among 100 foods commonly consumed in the United States (45). However, in the majority of studies, the total anthocyanin content is reported rather than amounts of individual anthocyanins. This approach may change because the bioavailability and health effects of anthocyanins seem to be affected by the structures of the aglycones or the glycosidic moieties (45–47).
Phenolic acids.
Cranberry also contains phenolic acids, including hydroxybenzoic and hydroxycinnamic acids. The former are the most abundant, with very high contents of benzoic acid at 474–557 mg/100 g FW (48–50) and much lower contents of 2,4-dihydroxybenzoic, p-hydroxybenzoic, and o-hydroxybenzoic acids at 2–4 mg/100 g FW (Fig. 1). The main hydroxycinnamic acids in cranberry are p-coumaric, sinapic, caffeic, and ferulic acids, with contents ranging from 8.8 to 25 mg/100 g FW (48). Of course, these phenolic acids are not specific to cranberries. A comparison of the phenolic acid content in cranberries with other berry fruit is difficult. Ellagic acid and ellagitannins have not been detected in significant amounts in American cranberry, whereas they are abundant in berries of the genus Rubus (raspberry, cloudberry) and Fragaria (strawberry).
Terpenes.
Although much less studied than the polyphenol composition, the presence of potentially active terpenes in cranberry deserves further attention. Ursolic acid (Fig. 1) is abundant in American cranberry at 46–109 mg/100 g FW (51). This triterpene is a constituent of numerous traditional herbal medicines and has strong anti-inflammatory effects (52). Ursolic acid is present in a limited range of foods (apple, guava, olive, several herbs). Cranberry also contains 2 rare derivatives of ursolic acid: cis-3-O-p-hydroxycinnamoyl ursolic acid (12–16 mg/100 g FW) and trans-3-O-p-hydroxycinnamoyl ursolic acid (42–60 mg/100 g FW) (Fig. 1). The iridoids, monotropein and 6,7-dihydromonotropein, have also been described in cranberry. An analysis of the fractionation of cranberry juice guided by a bacterial antiadherence assay revealed the presence of 2 new coumaroyl iridoid glycosides, 10-p-trans- and 10-p-cis-coumaroyl-1S-dihydromonotropein, as well as a depside, 2-O-(3,4-dihydroxybenzoyl)-2,4,6-trihydroxyphenylmethylacetate (53).
Flavonols.
Flavonols in cranberries consist mainly in glycosides of quercetin, myricetin, and to a lesser extent, kaempferol (Fig. 1). Quercetin 3-galactoside is the predominant form, but at least 11 other glycosides are present in lower concentrations (26, 41). Some of these, such as quercetin-3-acetylrhamnoside are rare in berries (54). As shown in the PhenolExplorer database, the flavonol content of plant foods is usually <3 mg/100 g FW (55), although bilberry, blackberry, and blueberry contain 3.2–17 mg/kg (54, 56, 57). In comparison, American cranberries have been described in several surveys as the richest fruit source for flavonols, containing 20–40 mg/100 g FW (26, 56). A comprehensive study in which flavonols were quantified in 28 wild and cultivated berry species revealed that elderberry and a few other berries that are consumed only in processed forms are richer in flavonols, at 23–57 mg/100 g FW, than cranberry (54).
Effect of food processing on cranberry bioactives.
Cranberry is rarely consumed fresh, due to its tart and astringent taste. It is chiefly consumed as processed juice (60%) and to a lesser extent as sauce and sweetened, dried fruit (48, 58). Multiple-step processing for juice production leads to a substantial loss of phytochemicals through elimination of rich fractions (skin, seeds), thermal degradation, as well as oxidation by polyphenol oxidase and peroxidase, so that compounds are retained to varying extents in processed foods. Anthocyanins are the most affected, with losses of >50%. Flavonols and PACs are somewhat heat-stable and resistant to the clarification and pasteurization steps but can be affected by high heat, which is sometimes used when cranberries are processed into powders. However, losses of 30–40% can occur during pressing, through removal of skin and seeds. In addition, the juice is often diluted or blended with other fruit juices, which diminishes or modifies the beverage phytochemical content. Nonetheless, cranberry juice can still contribute significantly to the intake of PACs and flavonols and, to a lesser extent, anthocyanins. Interestingly, the content of ursolic acid in sweetened, dried cranberries was found to be comparable to that measured in the fresh fruit (51).
Metabolomic approach to composition of fruit bioactives.
Because phytochemicals comprise an extraordinary diversity of structures, few studies have analyzed a wide range of cranberry phytochemicals at once. The emergence of nontargeted metabolomics opens a new avenue for the comprehensive description of plant food composition. A metabolomics approach allowed the identification of phytochemicals in tomatoes and strawberries, which had never been previously reported (59, 60). A nontargeted metabolomics analysis has been performed on 5 cranberry cultivars to compare their phytochemical diversity. Between 4477 and 6330 compounds were detected in each cultivar, with a large majority conserved in all cultivars (44).
Cranberries in Urinary Tract Health
Traditionally, urinary tract health has been managed through infection-prevention practices, including use of low-dose antibiotics. However, this approach carries a serious potential for the development of antibiotic resistance, so alternative strategies could have an important consequence for public health (61). Increasing fluid intake and acidifying the urine revealed inconsistent results in research trials (62, 63), although an early study reported increased urinary acidity “by eating prunes and cranberries” (64). Historically, plants (especially their polyphenols) have been used in herbal and traditional medicines (65). Because their antimicrobial activity is based on a number of different mechanisms of action, and the compounds responsible for the effects are often complex, it is unlikely that bacteria will develop resistance to them (66). The most widely studied alternative treatment is cranberry, which has been shown to be effective at preventing UTIs in a number of human intervention trials.
Mechanisms of action
Several mechanisms have been proposed for the actions of cranberry in the prevention of UTIs, with attention especially on the interference with bacterial adhesion in the urinary tract (67). An antiadhesion response is elicited in urine after cranberry consumption, preventing uropathogenic P-fimbriated E. coli from adhering to bladder cell receptors (68). If the bacteria cannot adhere to cells, they will not grow and cause infection. The reduction in adhesion forces (69) may be due to changes in bacterial morphology (70) and/or genetically based decreases in P-fimbrial expression (70, 71). In addition, a beneficial effect of cranberry bioactives on gut microbiota has been proposed, especially for the larger oligomer polyphenols (72). However, these putative mechanisms found in vitro have not been demonstrated in clinical efficacy trials.
Clinical trials
Whereas the effects of cranberry bioactives on bacterial-epithelial cell binding and pilus expression have been demonstrated in laboratory models, clinical evidence of a beneficial effect on human health has been difficult to elicit consistently. Observational studies associating cranberry consumption with UTIs have not been undertaken. A number of human intervention studies have been conducted with cranberry products, but synthesizing the information from these studies remains challenging, partly because different study designs and clinical conditions have been examined, different endpoints or effect markers have been measured, and different and unstandardized products have been used. The choice of study participants is particularly important because the pathogenesis of UTI is specific to different patient groups. Young, sexually active women often develop UTI after intercourse because bacteria can be introduced into the bladder during sex. Young children may develop UTI as a result of structural abnormalities in the urinary tract that predispose them to turbulent urinary flow and introduction of bacteria from the periurethral area to the bladder, renal pelvis, and/or kidney. Older women with recurrent UTIs differ from these other groups. Thus, to fully evaluate the potential of cranberry to prevent or treat UTIs, one must consider the individuals, product used, the dose and method of administration, length of exposure, compliance with regimen, and choice of comparator agent. Given these variables, it is not surprising that the results of individual studies and meta-analyses have been inconsistent (25).
In the first randomized double-blind study examining whether cranberry juice consumption could prevent the recurrence of UTI, women in a nursing home consumed 300 mL/d of artificially sweetened cranberry juice for 6 mo (73). After 1 mo, the prevalence of bacteriuria with pyuria was significantly lowered for the women who had consumed cranberry juice.
Although several subsequent studies have been conducted, the results have not been easy to interpret in all cases. One issue may be that compliance is easier to monitor in participants in a long-term care facility than in free-living participants in the general population. With the exception of 2 studies in elderly persons in hospital or long-term care facilities (73, 74), most other studies have been performed in participants living in the community, where monitoring of compliance is more difficult. Differences in the size of the study, study design (crossover vs. parallel, double-blinded and/or placebo-controlled), type of cranberry product and source (juice, tablet, supplier, etc.), dose, duration, washout period, and control of the participants’ diets (or absence thereof) during the intervention period all may affect study outcome.
Illustrating these challenges, 2 recent comprehensive reviews and a meta-analysis of the effect of cranberry consumption on UTIs each drew different conclusions. Wang et al. (5) initially examined 13 trials with a total of 1616 participants and subsequently performed a more detailed analysis of 10 trials (1494 participants) using quantitative methods. They concluded that consumption of cranberry products protected against UTIs and that an enhanced positive outcome was seen particularly among certain subgroups, namely women with recurrent UTIs and individuals who consumed cranberry products more than twice daily. In contrast, Jepson et al. (75) collected data from all randomized controlled trials or randomized controlled trials of cranberry products for the prevention of UTIs. Their analysis included 24 studies with a total of 4473 participants. They concluded that cranberry products did not significantly alter the occurrence of UTIs in the overall population or in any subgroups (women with recurrent UTI, pregnant women, children with recurrent UTI, cancer patients, or people with neurogenic bladder or spinal injuries). However, the calculated relative risk of developing UTIs in the treated versus control groups was <1.0. Risk ratios of <1.0 were interpreted as positive outcomes by Wang et al. (5) but not by Jepson et al. (75) In addition, different CIs were reported in each study.
Clinical trials in adult women and in women with recurrent UTIs.
Women, who have a higher risk of UTIs than men, and particularly women with recurrent UTIs have been studied most frequently in cranberry interventions. An early case report considered a 66-y-old woman who had chronic pyelonephritis that was not treatable with antibiotics (76). After 8 wk of treatment with 180 mL cranberry juice twice daily, there was an improvement in her urine (determined by albuminuria and pyuria), and the infection was almost completely cleared after 9 mo. She did not require antibiotics again for 2.5 y.
Placebo-controlled trials have examined women with a history of recurrent UTIs to determine whether cranberry consumption can prevent outbreaks and included both cross-over (77) and parallel (78–80) designs. Different cranberry products and placebos were administered in these trials, with only 1 study (80) using the placebo specifically designed for cranberry juice by the NIH National Center for Complementary and Alternative Medicine.
Walker et al. (77) used 400 mg/d of encapsulated cranberry solids or placebo for 3 mo in a group of 19 patients and found a diminution in the recurrence of UTIs during the active treatment period. In a trial in 150 women with recurrent UTI, Stother (79) tested pure cranberry juice, concentrated cranberry extract, and placebo and found the UTI incidence to be 30%, 39%, and 72% in each group, respectively. In contrast, Barbosa-Cesnik et al. (78) demonstrated no diminution in the recurrence of UTIs with cranberry treatment (240 twice daily) compared with placebo in 319 college women but obtained recurrence rates of 19.3% and 14.6%, respectively, that were much lower than the 30% rate anticipated, so the statistical power of the study appeared to be compromised. Stapleton et al. (80) randomly assigned 176 young women to receive either 120 or 240 mL/d of cranberry juice or a placebo beverage. The overall result showed a nonsignificant decrease of 32% in the risk of UTI for women who consumed the cranberry beverages compared with placebo. Notably, this study directly found a correlation between the clinical outcomes and the in vitro determination of P-fimbriated E. coli, consistent with the mechanism proposed by Howell et al. (67) of prevention of recurrent UTIs via interference by cranberry bioactives of the binding of P-fimbriated E. coli to bladder epithelial cells.
There are other studies in women that have shown positive results, although there are weaknesses in the study designs. Testing 150 women with previous UTIs, Kontiokari et al. (81) examined 50 mL of cranberry-lingonberry juice versus 100 mL of Lactobacillus GG using a “no treatment” control. Absent a placebo and a standardized cranberry product, they reported a 20% lowering in UTIs for the women who consumed the cranberry-lingonberry beverage. Foxman et al. (82) evaluated the relation between UTIs and sexual behavior in sexually active women with no prior UTI history, a unique population among cranberry studies. Data on cranberry juice and carbonated soft drink consumption were collected as part of the study, but these were not prescribed treatments. They found that although vaginal intercourse increased the risk of UTIs, adjusting for this increase revealed that cranberry juice was protective against UTI, whereas carbonated soft drinks were associated with an increased risk of UTIs.
Clinical trials in children.
Ferrara et al. (83) examined 84 girls aged 3–14 y who had experienced >1 UTI in the past 12 mo in a placebo-controlled parallel design study. Participants were given 50 mL of cranberry-lingonberry concentrate daily, 100 mL of Lactobacillus GG administered 5 d/mo, or a “no treatment” control for 6 mo. The UTI rate was 18.5% for the group that consumed cranberry-lingonberry juice, 42.3% for the Lactobacillus group, and 48.1% for the control group.
Foda et al. (84) enrolled children with an average age of 9.4 y and neuropathic bladders undergoing intermittent catheterization in a crossover trial and tested 15 mL/(kg · d) of cranberry juice cocktail or water for 6 mo. No difference was seen in either asymptomatic bacteriuria or UTI recurrence between the 2 groups. However, 19 of the 40 participants withdrew during the study period, limiting the study power to show a statistically significant effect. By using a parallel trial design, Salo et al. (85) tested 255 children with a previous UTI diagnosis with cranberry juice [5 mL/(kg · d); up to 300 mL] or a placebo juice for 6 mo. Whereas cranberry treatment did not decrease the number of children who experienced a recurrent UTI, there was a trend showing a lowering in the number of recurrent UTIs and a reduction of 34% in the number of days per patient-year of antibiotic treatment. These investigators also found that compliance was better for the placebo group (80% of doses consumed) than for the treatment group (64% of doses consumed), showing the critical importance of using products and dosing regimens that are palatable for the participants and carefully monitoring participant compliance. In a randomized controlled trial, Afshar et al. (86) treated 39 girls and 1 boy (median age of 7 y) with at least 2 documented nonfebrile UTIs in the calendar year before enrollment with cranberry juice containing either a high PAC content (37%) or with no PAC. After 1 y of follow-up, the average incidence of UTIs was 0.4 and 1.15 in the treatment and placebo groups, respectively, representing a statistically significant 65% lowering of the risk of UTI. Thus, to date, there are now 3 independent randomized trials demonstrating a beneficial effect of cranberry juice in children with recurrent UTIs.
Effects from other Vaccinium berry fruit bioactives on urinary tract health
As discussed above, there is some overlap in the bioactives profile between cranberry and other Vaccinium species, particularly highbush blueberry (Vaccinium corymbosum L.), lowbush or “wild” blueberry (Vaccinium angustifolium Aiton), and lingonberry (V. vitis-idaea L.) (87, 88). For example, the polymerized PACs in blueberry (89) and lingonberry (32, 90) have been found to contain A-type linkages, albeit with different profiles and amounts than those found in cranberries.
Schmidt et al. (88) reported a significant positive correlation between the oligomeric PAC content of different fractions of wild blueberry and in vitro bioactivity, with fractions containing higher molecular weight PACs inhibiting the adhesion of P-fimbriated E. coli most effectively. Similarly, Ofek et al. (91, 92) found that polyphenols from blueberry but not grapefruit, guava, mango, orange, or pineapple prevented P-fimbriated bacterial adhesion in vitro. Clinical trials of the effect of blueberry urinary tract health have not been performed to date.
Cranberries in Cardiovascular Health
Mechanisms of action
A variety of mechanisms might account for a favorable effect of cranberry consumption on cardiovascular disease (CVD), including effects on CVD risk factors such as dyslipidemia, diabetes, hypertension, inflammation, oxidative stress, endothelial dysfunction, arterial stiffness, and platelet function. Such effects might slow atherogenesis, lesion progression, plaque rupture, thrombosis, myocardial infarction (MI), and the subsequent development of ischemic cardiomyopathy.
Dyslipidemia.
Animal and human studies suggest that consumption of cranberry juice and cranberry anthocyanins lowers LDL-C and increases HDL-C. For example, consumption of cranberry bioactives improved lipid profiles in Golden Syrian hamsters fed a high-fat diet (93), ovariectomized rats (94), and hypercholesterolemic swine (95). With regard to clinical studies, a placebo-controlled clinical trial involving 150 participants with hypercholesterolemia showed that consumption of a purified mixture of anthocyanins (320 mg/d) lowered LDL-C and increased HDL-C (96). Favorable effects of cranberry juice on blood lipids have also been demonstrated in other populations, including in obese men (14), patients with diabetes mellitus (13), and patients with low HDL-C and hypertriglyceridemia (97). Other studies examining healthy volunteers and patients with CVD failed to show significant effects of cranberry juice consumption on plasma lipids (15, 16, 18, 19, 24), but these discrepant results likely relate to differences in study populations, baseline lipids, and background medications, including lipid-lowering therapy.
The precise mechanism that might account for an improved lipoprotein profile after consumption of cranberry bioactives is incompletely understood. Studies with cultured hepatocytes indicate that a cranberry extract can increase the surface expression of LDL receptors and uptake of LDL-C (98), which would be expected to lower plasma LDL-C concentrations. A clinical study in patients with dyslipidemia suggests that anthocyanin supplementation inhibits cholesterol ester transfer protein (CETP) (97), which would be expected to increase HDL-C concentrations and enhance reverse cholesterol transport, although it currently remains controversial whether CETP inhibition actually lowers cardiovascular risk (99).
Diabetes and hypertension.
The data supporting an effect of cranberry bioactives on other CVD risk factors such as diabetes mellitus and hypertension are less strong. There is evidence that an intravenous infusion of dilute buffered cranberry juice lowers blood pressure in anesthetized rats (100). Cranberry extract also prevented an increase in blood pressure associated with consumption of a high-fat diet in Golden Syrian hamsters (93). An in vitro study suggested that cranberry extracts inhibit angiotensin converting enzyme and thus might be expected to lower blood pressure (101). To date, clinical studies in patients with diabetes mellitus and CVD have failed to show blood pressure–lowering effects after the consumption of cranberry juice (13, 15, 16). A study examining the effects of anthocyanin supplementation (320 mg/d for 4 wk) also showed no effect on ambulatory blood pressure in untreated hypertensive patients (102).
Diabetes is a potentially modifiable risk factor for CVD that might be affected by cranberry bioactives. Animal studies have demonstrated that cranberry powder or cranberry-derived flavonoids lower blood glucose and improve insulin sensitivity in models of diabetes mellitus (103, 104). Cranberry supplementation, however, had no effect on glycemic control in patients with type 2 diabetes mellitus (13, 105).
Oxidative stress.
Increased oxidative stress and oxidative modification of lipids, proteins, and nucleic acids contribute to the pathogenesis of atherosclerosis and other forms of CVD (106). These data are convincing, despite the findings of randomized clinical trials that showed no benefit of relatively high doses of a few antioxidant vitamins. This apparent discrepancy has been attributed to the important physiologic signaling role played by reactive oxygen species (ROS) in the vasculature as well as the potentially harmful effects of indiscriminant ROS scavengers. It also has been suggested that inhibiting enzymatic sources of ROS is a more effective strategy to lower oxidative stress, providing a partial explanation for the proven benefits of certain interventions, including statins and angiotensin converting enzyme inhibitors (107). There is a large body of work showing that cranberry bioactives have antioxidant effects in vitro and in vivo in experimental models, and it seems plausible that such effects might contribute to benefits from cranberry consumption (7, 108).
Although (poly)phenols are often defined as dietary antioxidants, their principal mechanisms of action appear to be unassociated with directly reducing ROS (109). Nonetheless, there is reasonable evidence that consumption of cranberry juice or cranberry bioactives diminishes blood markers of oxidative stress in healthy volunteers and in patients with cardiovascular risk factors. For example, Duthie et al. (19) showed that consumption of cranberry juice (750 mL/d for 2 wk) improved plasma antioxidant capacity in healthy female volunteers. Increases in plasma antioxidant capacity and a decrease in circulating concentrations of oxidized LDL-C have been reported in healthy men (18) and in sedentary men (110). Basu et al. (24) observed a similar effect of cranberry juice on antioxidant capacity and circulating oxidized LDL-C in women with metabolic syndrome. However, most studies to date have failed to provide evidence for an actual decrease in oxidative damage to lipids or nucleic acids, as reflected by biomarkers such as F2-isoprostanes or 8-hydroxydeoxyguanosine. Thus, it remains uncertain whether the beneficial effects of cranberry bioactives on vascular function or CVD can be attributed simply to their ability to scavenge ROS.
Inflammation.
Atherosclerosis is an inflammatory disease, and there is growing appreciation that intervention-induced changes in CRP and other biomarkers of inflammation provide surrogate information about cardiovascular risk (111). For this reason, there is considerable interest in the possibility that consumption of cranberry bioactives will have anti-inflammatory effects and decrease concentrations of inflammatory cytokines. Several in vitro studies suggest that cranberry bioactives suppress the activation of macrophages and T cells exposed to relevant proinflammatory stimuli (112, 113). Notably, this mechanism has also been suggested to contribute to the favorable effects of cranberry juice against periodontal disease (114). Cranberry anthocyanins have a similar effect on microvascular endothelial cells and act to blunt expression of intercellular adhesion molecule 1 (ICAM-1) and monocyte chemotaxic protein 1 after exposure to proinflammatory cytokines (115). Anthocyanins also prevent hyperoxia-induced activation of nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) and other proinflammatory pathways in cultured endothelial cells (116).
Several human studies provide evidence for an anti-inflammatory effect of cranberry bioactives. Ruel et al. (110) showed that consumption of cranberry juice reduced circulating adhesion molecules in sedentary middle-aged men with risk factors. Zhu et al. (96) reported that consumption of a purified mixture of anthocyanins (320 mg/d) led to significant decreases in CRP, soluble vascular cell adhesion molecule 1 (sVCAM-1), and plasma IL-1β in patients with hypercholesterolemia. In contrast, several studies failed to demonstrate significant effects on CRP, adhesion molecules, and other plasma markers of inflammation (13, 15, 16, 24). As for studies of plasma lipids, differences in participant population and background medications may account for the lack of a detectable anti-inflammatory effect in some studies, but further studies are needed to explain these discrepant findings.
Endothelial dysfunction.
The vascular endothelium maintains homeostasis via production of many factors that act locally in the vascular wall and lumen to control vasomotor tone, thrombosis, inflammation, and angiogenesis (117). Endothelium-derived NO plays a particularly important role by acting as a vasodilator, an antiplatelet and anti-inflammatory factor, and by inhibiting the proliferation of vascular smooth muscle cells and promoting angiogenesis. Loss of NO contributes to atherogenesis, lesion progression, and risk of cardiovascular events (117, 118). Many interventions that restore the bioavailability of NO are known to lower cardiovascular risk, including cholesterol-lowering drugs, angiotensin converting enzyme inhibitors, behavioral interventions such as weight loss and exercise, and dietary interventions such as flavonoid-containing foods and beverages.
Experimental studies have shown favorable effects of cranberry bioactives on endothelial function and NO bioavailability. Exposure of isolated rat arterial rings to cranberry juice extract enhanced endothelium-dependent vasodilation (100). Treatment of ovariectomized rats with cranberry juice improved endothelium-dependent dilation and increased concentrations of activated endothelial NO synthase (eNOS) in isolated aortic tissue (94). These changes were associated with decreased expression of NADPH oxidase, a source of superoxide production, suggesting that improved endothelial vasodilator function in this setting might be related to a decrease in oxidative stress. A cranberry juice extract was shown to induce phosphorylation of eNOS at serine 1177 via PI3 kinase/Akt signaling in cultured endothelial cells, a modification associated with increased NO production (119). Anthocyanins found in cranberries also improve endothelial vasodilator function. For example, malvidin-3-glucoside was reported to enhance expression of eNOS and NO production and to have anti-inflammatory effects in cultured endothelial cells (120).
Clinical studies of cranberry bioactives and endothelial vasodilator function have provided mixed results. In a crossover study comparing the effects of anthocyanins to placebo, Zhu et al. (12) observed an acute improvement in endothelium-dependent dilation 1–2 h after consumption in patients with hypercholesterolemia. In contrast, Dohadwala et al. (15) observed no effect of cranberry juice consumption (480 mL/d providing 835 mg/d total polyphenols for 4 wk) on brachial artery flow-mediated dilation or fingertip peripheral arterial tonometry [measured by EndoPAT (Itamar Medical, Caesarea, Israel)] in patients with coronary artery disease. That study examined vasodilation over 12 h after the last cranberry juice consumption, a time when cranberry polyphenols would be expected to be low or undetectable in plasma (121). Consistent with the acute study by Zhu et al. (12), Dohadwala et al. (15) observed improved endothelial vasodilator function 2–4 h after cranberry juice consumption in an uncontrolled pilot study, but a placebo-controlled trial would be required to confirm those findings. Flammer et al. (16) also observed no significant improvement in endothelial function measured by EndoPAT after consumption of double-strength cranberry juice (460 mL/d) in patients with risk factors and endothelial dysfunction at baseline. However, cranberry juice consumption was associated with a favorable effect on the phenotype of circulating endothelial progenitor cells, which play a role in restoring vascular function after vascular injury. As with other polyphenol interventions, favorable effects of cranberry juice on endothelial vasodilator function appear to be evident a few hours after acute consumption, at a time that corresponds to peak plasma concentrations of cranberry bioactives (122, 123).
Arterial stiffness.
Stiffness of the central aorta is increasingly recognized as an important aspect of vascular function relevant to the pathogenesis of hypertension and heart failure (124, 125). Arterial stiffness is influenced by structural factors, including the relative amounts of elastin and collagen in the arterial wall, and by dynamic factors such as arterial tone and the balance of vasodilators and vasoconstrictors produced locally (126). Measures of arterial stiffness, particularly carotid-femoral pulse wave velocity (PWV), predict cardiovascular events and incident hypertension and respond to dietary and pharmacologic interventions (124–126).
In a placebo-controlled crossover clinical trial involving 44 patients with coronary artery disease, Dohadwala et al. (15) demonstrated that consumption of cranberry juice (480 mL/d of 54% juice providing 835 mg total polyphenols/d) for 4 wk significantly decreased central aortic stiffness as measured by carotid-femoral PWV. In contrast, Ruel et al. (127) observed no effect of cranberry juice consumption (500 mL/d of 27% juice providing 400 mg total polyphenols/d) for 4 wk on augmentation index in obese men, although there was a trend for an effect in the subgroup with metabolic syndrome. Although differences in study population and amount of juice might explain the discrepant results, it is notable that PWV relates strongly to cardiovascular events, whereas augmentation index does not (124), suggesting that PWV has greater clinical relevance.
Platelet function.
The importance of platelet aggregation for acute cardiovascular events, such as unstable angina and acute MI, is well recognized; antiplatelet agents such as aspirin and clopidogrel lower cardiovascular risk. Other polyphenol-containing beverages are known to inhibit platelet aggregation, including tea and grape juice (128, 129), and there is interest in the possibility that cranberry bioactives might have similar effects. Although no clinical study has examined the effects of cranberry juice consumption on platelet function, Yang et al. (130) showed that delphinidin-3-glucoside, an anthocyanin found in cranberry juice, significantly inhibited platelet activation in isolated platelets and inhibited thrombosis in a mouse carotid injury model.
Clinical trials
As discussed above, a growing body of clinical research has examined whether cranberry phenolics may lower the risk of CVD via modulation of multiple risk pathways using doses of these bioactives that are achievable with daily consumption of most cranberry products (Supplemental Table 1). Most studies have examined commercially available juice containing 27% cranberry juice at doses of ≤750 mL/d or 54% cranberry juice at doses of ~500 mL/d (131, 132). No studies conducted to date have used a 100% cranberry juice intervention. Lee et al. (13) used a powdered cranberry juice concentrate but did not provide equivalency information to the whole fruit or juice. A 240-mL glass of 100% pure cranberry juice (70 kcal) daily approximately would provide a dose of cranberry juice that is equivalent or greater than the doses examined in clinical studies to date.
Oxidized LDL-C was not improved in a crossover study in 35 overweight men after consuming 500 mL/d of cranberry juice for 4 wk (127). However, in a placebo-controlled study in individuals with metabolic syndrome (15–16/group) given 480 mL/d of cranberry juice for 8 wk, plasma antioxidant capacity, oxidized LDL-C, and malondialdehyde were significantly improved (24). It is possible that a relatively longer supplementation period and/or presence of modifiable risk factors are needed.
Shidfar et al. (133) reported improvement in apo B, apo A-1, paraoxonase 1, and glucose concentrations after 240 mL/d cranberry juice in a randomized trial in 58 men with type 2 diabetes but did not report a traditional lipid panel; Lp(a), an atherogenic form of LDL-C, was not changed. Thus, there is inconsistent evidence regarding the effects of cranberry juice consumption on lipids and lipoproteins. Nonetheless, given that some studies reported beneficial effects, further studies are warranted to clarify whether, and the extent to which, cranberry juice affects lipids and lipoproteins, as well as associated blood markers of CVD risk.
Ruel et al. (110, 134) reported a reduction in matrix metalloproteinase 9, ICAM-1, and sVCAM-1 at the end of their dose escalation trial coinciding with the 500 mL/d dose of cranberry juice. However, in the placebo-controlled study by Flammer et al. (16), ICAM-1 and sVCAM-1 were not lowered by cranberry juice. In a randomized crossover study, Ruel et al. (127) tested 480 mL/d unsweetened cranberry juice for 4 wk in 35 overweight men and found no difference in augmentation index, blood pressure, or adhesion markers between groups. However, these researchers highlighted a significant 14% decrease in augmentation index relative to baseline after the cranberry juice intervention. Blood pressure results have been inconsistent among studies, with little emphasis on this endpoint (15, 16, 24, 110, 127, 134). Further studies are needed to evaluate whether cranberry juice consumption improves measures of arterial stiffness and function, as well as blood pressure.
Cranberry juice has been shown to increase plasma and urinary concentrations of salicylic acid (135), which may affect enzymatic pathways activated during inflammatory responses, providing a provocative mechanism for future clinical studies that make use of an inflammatory stimulus.
Observational studies
In contrast to the absence of observational studies looking at the association between cranberry intake and urinary tract health, there is a growing body of such evidence with regard to the intake of (poly)phenols that are found in cranberries (and other plant foods) and cardiovascular health.
Anthocyanins.
Several prospective cohort studies have examined the associations between habitual anthocyanin intakes and CVD outcomes (Supplemental Table 2) or biomarkers of CVD risk (Supplemental Table 3), predominantly in U.S. populations. Coronary heart disease (CHD) and nonfatal MI were examined in 3 studies, with evidence suggesting that increased anthocyanin intake is significantly associated with a lowered risk of CHD by 12–32% in multivariate analyses (136–138). The magnitude of the protective effect of increased anthocyanin intake was smaller (12–21%) in older women and men compared with the 32% risk reduction seen in younger and middle-aged women comparing extremes of anthocyanin intake (138). Median intakes in these younger/middle-aged women were 12 mg/d. When extreme deciles of intake were compared, those in the top decile had a 47% lowering in risk of MI; for every 15-mg increase in anthocyanin intake, the relative risk of MI decreased by 17% in the multivariate model, suggesting a continual dose-response at higher intakes. The relation between anthocyanin intake and CVD mortality was also examined in several studies, with 1 study showing no association (139), whereas others observed a 9–14% decrease in risk comparing higher with lower intakes (136, 137). The impact of increased anthocyanin intake on stroke has been examined, but there is currently no evidence for an association (136, 137, 139, 140).
In relation to CVD risk biomarkers, prospective studies and cross-sectional data provide mechanistic support for the observed decrease in CHD risk with increased anthocyanin intake. Specifically, higher intakes improve arterial stiffness (as assessed by PWV) and blood pressure, but the limited data on the effects on inflammatory biomarkers are equivocal. Interestingly, the greatest decrease in hypertension was observed in younger/middle-aged women, supporting the observed decrease in risk of MI in this age group (138, 140). The magnitude of the associations observed between anthocyanin intake and systolic blood pressure (−4 mm Hg decrease with higher intake) was similar to that previously reported for smoking cessation and 2-fold higher than that observed after a 1.4-portion increase in fruit and vegetable intake (142).
Flavonols.
A meta-analysis of prospective cohort studies published before 2002 suggests that increased intake of flavonols was associated with a 20% decrease in CHD mortality (143). However, a more recent systematic review of studies published through January 2012 observed no significant association when all the prospective cohort data were combined, when analyses were restricted to the 5 most recent studies, and when a dose-response analysis was conducted (144). The earlier studies had relied on less comprehensive flavonoid compositional databases, which have improved significantly over the past decade. In several of these studies, a distinction was not made between flavonols and another subclass, flavones, although flavones do not contribute significantly to habitual dietary intake of total flavonoids (<5 mg/d) (145). Since the 2012 publication of the systematic review by Wang et al. (144), 2 other studies have examined the associations between habitual flavonol intake in U.S. populations and risk of CHD, with adjustment for CHD risk factors showing trends toward a decrease in risk but without statistical significance (137, 138).
In relation to risk of stroke, a meta-analysis that included all prospective cohort data through August 2009 provided evidence to suggest a protective effect of increased flavonol intake on risk of stroke (145). However, 2 more recent U.S.-based population studies do not support this observed decrease in risk (137, 140). Interestingly, in the 2 studies that examined CVD mortality, 1 observed no effect (139), whereas a more recent study observed a 16% decrease in risk with increased flavonol intake (Supplemental Table 2) (137).
In relation to biomarker studies, in older women there was a small decrease in risk of hypertension with increased flavonol intake (4%) but no association in middle-aged women or in men (141). In a cross-sectional study, Mursu et al. (146) observed a small trend toward a decrease in intima-media thickness (IMT), an indicator of the presence of carotid atherosclerosis and an independent predictor of CVD, in middle-aged men with high habitual flavonol intake. However, in another study, increased flavonol intake was not associated with IMT, PWV, blood pressure, or other measures of vascular health (142). In 2 studies that assessed inflammatory biomarkers, 1 observed a reduction in CRP concentrations (147) whereas the other observed no effect on CRP or a number of other biomarkers but did report a significant decrease in sVCAM-1 concentrations (148).
Flavan-3-ols.
Five prospective cohort studies have examined the associations between habitual flavan-3-ol intake and CHD risk (136–138, 149, 150), but only 1 study showed a significant association between increased flavan-3-ol intake and a 51% decrease in risk of CHD mortality comparing the highest with the lowest tertile of intake (150). However, in the same study, a higher flavan-3-ol intake was not inversely associated with CHD incidence. In 2001, because no comprehensive database for assessing the flavonoid content of the habitual diet was available, analyses of >120 commonly consumed plant foods and beverages from the Dutch diet were used to construct one (149, 150).
CVD mortality was investigated in 3 prospective cohort studies (136, 137, 139) and only the most recent one, using recent USDA flavonoid values (151), observed a 17% decrease in risk comparing the highest to the lowest quintile of flavan-3-ol intake (137). The impact of increased flavan-3-ol intake on stroke risk has been examined in 5 studies with no evidence of a protective effect (136, 137, 139, 140, 150).
With regard to biomarkers for CVD risk, the prospective and cross-sectional studies conducted to date do not show any significant associations between flavan-3-ol intake and blood pressure, inflammatory markers, PWV, or augmentation index (141, 142, 147, 148). Two studies examined the associations between flavan-3-ol intake and IMT with 1 study observing no association (142) and another observing a significant decrease in IMT in Finnish middle-aged men (146).
Proanthocyanidins/polymers.
Polymeric flavan-3-ols include PACs, as well as theaflavins and thearubigins. However, PACs are the main contributors to total flavonoid intake in American and European populations, but data on either the bioavailability or bioactivity of these compounds are limited (27, 37, 152–154). The main dietary sources of PACs in the U.S. habitual diet are tea, legumes, and wine (153), whereas thearubigins and theaflavins are mainly consumed with black tea (37, 155).
Of the 3 studies that have examined the effects of high polymer (138) or proanthocyanidin (136, 137) intake on CHD mortality or incidence, none showed any significant associations. Cassidy et al. (138) reported a 17% decrease in incident CHD comparing extremes of polymer intake in young/middle-aged women, which almost reached statistical significance. CVD mortality has only been investigated in 2 prospective cohort studies (136, 137). By using the most up-to-date versions of the USDA flavonoid database (32, 151), McCullough et al. (137) observed a 13% decrease in risk in participants consuming the highest proanthocyanidin intake. There was no observed association between high intakes of polymeric flavan-3-ols or PACs and risk of stroke (136, 137, 140).
Summary
Cranberries are a rich source of dietary phenolic bioactives, including, in particular, flavan-3-ols, A-type PACs, anthocyanins, benzoic acid, and ursolic acid and a unique profile of all 6 members of the anthocyanidin family. These compounds together with the very low natural sugar content of cranberries are responsible for their characteristic tart taste and astringency. For these reasons, sugar is often added to cranberry products to an amount found in other fruit juices and dried-fruit products: e.g., the total sugar content of sweetened cranberry juice is 11.7 g/100 mL compared with 100% purple grape juice, apple juice, and orange juice at 16.5, 11.1, and 10.5 g/100 mL, respectively (156). Nonetheless, cranberry products can fit readily within the calorie, total fat, saturated fat, trans fat, and sodium recommendations of the 2010 Dietary Guidelines for Americans (1). Indeed, these guidelines state that the best use of calories from added sweeteners is to increase the palatability of nutrient-dense foods, e.g., the addition of sugar to fruit. In addition, artificial sweeteners are used to produce low-calorie versions of cranberry products.
To date, several studies and 2 meta-analyses indicate a benefit of cranberry intake in lowering the recurrence of UTIs. However, the number of studies finding null results on this outcome reveals the complexity in studying the relation between cranberry consumption and health outcomes. Specifically, interventions testing the efficacy of cranberry juice are confounded by poor compliance, dropout rates as high as 50%, and variable doses of bioactives from the use of different products. Another confounding factor is the apparent difference in compliance among cranberry products versus placebo. When dropout rates are high and/or the degree of compliance is unknown, the accurate interpretation of study results is seriously compromised. Furthermore, the optimal dose of cranberry bioactives has not been determined for urinary tract or cardiovascular health. For example, there appears to be an increasing trend in the reduction in UTIs with higher cranberry juice intake, but very few studies have addressed such dose-response relations in a systematic way. Another major issue with the evaluation of existing clinical studies is the lack of quantification of cranberry bioactives in the product or assessment of their concentration in blood or urine. Although the NIH developed a standardized cranberry juice placebo in 2003, very few published studies have made use of the product.
There is strong experimental evidence that cranberry bioactives have favorable effects on blood pressure, glucose metabolism, lipoprotein profiles, oxidative stress, inflammation, and endothelial function. However, the currently available data from human studies provide mixed results about the clinical significance of these actions on cardiovascular health. The evidence for in vivo effects on oxidative stress and inflammation is not convincing at this stage. Favorable effects on endothelial function appear to be limited to acute responses after consumption of cranberry juice or cranberry anthocyanins. One well-controlled study suggests a chronic benefit on carotid-femoral PWV, which is emerging as an important measure of arterial function with relevance to the risk of CVD (15). Thus, there is encouraging, but limited, evidence of cardioprotective effects of cranberries.
As noted, the average daily consumption of fruit by Americans is substantially less than recommended by the 2010 Dietary Guidelines for Americans (1). In part, encouraging a greater proportion of plant foods, including fruit, to achieve a healthy dietary pattern is targeted to help us meet the Recommended Dietary Intakes of micronutrients. Although reference intake values have yet to be developed for phytochemicals, there is a growing consensus that these bioactives contribute importantly to promoting health and reducing the risk of chronic disease. Berry fruit, including cranberries, represent an especially rich source of many phenolic acids and flavonoids that have been associated with these benefits. More specific dietary guidance to choosing a broad array of types of fruit, including berry fruit, should help increase our intake of these bioactive compounds.
Acknowledgments
All authors read and approved the final manuscript. In addition to their contribution to the writing, J.B.B. and H.S. outlined and coedited this article. Pollock Communications (New York) helped format the final version of the manuscript.
Footnotes
Abbreviations used: CETP, cholesterol ester transfer protein; CHD, coronary heart disease; CRP, C-reactive protein; CVD, cardiovascular disease; DP, degree of polymerization; eNOS, endothelial NO synthase; FW, fresh weight; HDL-C, HDL cholesterol; ICAM-1, intercellular adhesion molecule 1; IMT, intima-media thickness; LDL-C, LDL cholesterol; MI, myocardial infarction; NF-κB, nuclear factor κ-light-chain-enhancer of activated B cells; PAC, proanthocyanidin; PWV, pulse wave velocity; ROS, reactive oxygen species; sVCAM-1, soluble vascular cell adhesion molecule 1; UTI, urinary tract infection.
Literature Cited
- 1.USDA; U.S. Department of Health and Human Services Dietary guidelines for Americans, 2010. 7th ed. Washington: U.S. Government Printing Office; 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paredes-López O, Cervantes-Ceja ML, Vigna-Pérez M, Hernández-Pérez T. Berries: improving human health and healthy aging, and promoting quality life—a review. Plant Foods Hum Nutr. 2010;65:299–308 [DOI] [PubMed] [Google Scholar]
- 3.Côté J, Caillet S, Doyon G, Sylvain JF, Lacroix M. Bioactive compounds in cranberries and their biological properties. Crit Rev Food Sci Nutr. 2010;50:666–79 [DOI] [PubMed] [Google Scholar]
- 4.Basu A, Lyons TJ. Stawberries, blueberries, and cranberries in the metabolic syndrome: clinical perspectives. J Agric Food Chem. 2012;60:5687–92 [DOI] [PubMed] [Google Scholar]
- 5.Wang CH, Fang CC, Chen NC, Liu SS, Yu PH, Wu TY, Lee CC, Chen SC. Cranberry-containing products for prevention of urinary tract infections in susceptible populations: a systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2012;172:988–96 [DOI] [PubMed] [Google Scholar]
- 6.Kaspar KL, Khoo C. Cranberry polyphenols in the promotion of urinary tract, cardiovascular and emerging health areas. In: Skinner M, Hunter D, editors. Bioactives in fruit: health benefits and functional foods. New York: Wiley Blackwell; 2013. p. 273–92. [Google Scholar]
- 7.McKay DL, Blumberg JB. Cranberries (Vaccinium macrocarpon) and cardiovascular disease risk factors. Nutr Rev. 2007;65:490–502 [DOI] [PubMed] [Google Scholar]
- 8.Del Rio D, Rodriguez-Mateos A, Spencer JP, Tognolini M, Borges G, Crozier A. Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal. 2013;18:1818–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim MJ, Ohn J, Kim JH, Kwak HK. Effects of freeze-dried cranberry powder on serum lipids and inflammatory markers in lipopolysaccharide treated rats fed an atherogenic diet. Nutr Res Pract. 2011;5:404–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yung LM, Wong WT, Tian XY, Leung FP, Yung LH, Chen ZY, Yao X, Lau CW, Huang Y. Inhibition of renin-angiotensin system reverses endothelial dysfunction and oxidative stress in estrogen deficient rats. PLoS ONE. 2011;6:e17437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao SD, Shi T. Is cranberry juice effective in the treatment and prevention of Helicobacter pylori infection of mice? Chin J Dig Dis. 2003;4:136–9 [Google Scholar]
- 12.Zhu Y, Xia M, Yang Y, Liu F, Li Z, Hao Y, Mi M, Jin T, Ling W. Purified anthocyanin supplementation improves endothelial function via NO-cGMP activation in hypercholesterolemic individuals. Clin Chem. 2011;57:1524–33 [DOI] [PubMed] [Google Scholar]
- 13.Lee IT, Chan YC, Lin CW, Lee WJ, Sheu WH. Effect of cranberry extracts on lipid profiles in subjects with type 2 diabetes. Diabet Med. 2008;25:1473–7 [DOI] [PubMed] [Google Scholar]
- 14.Ruel G, Pomerleau S, Couture P, Lemieux S, Lamarche B, Couillard C. Favourable impact of low-calorie cranberry juice consumption on plasma HDL-cholesterol concentrations in men. Br J Nutr. 2006;96:357–64 [DOI] [PubMed] [Google Scholar]
- 15.Dohadwala MM, Holbrook M, Hamburg NM, Shenouda SM, Chung WB, Titas M, Kluge MA, Wang N, Palmisano J, Milbury PE, et al. Effects of cranberry juice consumption on vascular function in patients with coronary artery disease. Am J Clin Nutr. 2011;93:934–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flammer AJ, Martin EA, Gossl M, Widmer RJ, Lennon RJ, Sexton JA, Loeffler D, Khosla S, Lerman LO, Lerman A. Polyphenol-rich cranberry juice has a neutral effect on endothelial function but decreases the fraction of osteocalcin-expressing endothelial progenitor cells. Eur J Nutr. 2013;52:289–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson T, Luebke JL, Morcomb EF, Carrell EJ, Leveranz MC, Kobs L, Schmidt TP, Limburg PJ, Vorsa N, Singh AP. Glycemic responses to sweetened dried and raw cranberries in humans with type 2 diabetes. J Food Sci. 2010;75:H218–23 [DOI] [PubMed] [Google Scholar]
- 18.Ruel G, Pomerleau S, Couture P, Lamarche B, Couillard C. Changes in plasma antioxidant capacity and oxidized low-density lipoprotein levels in men after short-term cranberry juice consumption. Metabolism. 2005;54:856–61 [DOI] [PubMed] [Google Scholar]
- 19.Duthie SJ, Jenkinson AM, Crozier A, Mullen W, Pirie L, Kyle J, Yap LS, Christen P, Duthie GG. The effects of cranberry juice consumption on antioxidant status and biomarkers relating to heart disease and cancer in healthy human volunteers. Eur J Nutr. 2006;45:113–22 [DOI] [PubMed] [Google Scholar]
- 20.Vinson JA, Bose P, Proch J, Kharrat HA, Samman N. Cranberries and cranberry products: powerful in vitro, ex vivo, and in vivo sources of antioxidants. J Agric Food Chem. 2008;56:5884–91 [DOI] [PubMed] [Google Scholar]
- 21.Gotteland M, Andrews M, Toledo M, Muñoz L, Caceres P, Anziani A, Wittig E, Speisky H, Salazar G. Modulation of Helicobacter pylori colonization with cranberry juice and Lactobacillus johnsonii La1 in children. Nutrition. 2008;24:421–6 [DOI] [PubMed] [Google Scholar]
- 22.Shmuely H, Yahav J, Samra Z, Chodick G, Koren R, Niv Y, Ofek I. Effect of cranberry juice on eradication of Helicobacter pylori in patients treated with antiobiotics and a proton pump inhibitor. Mol Nutr Food Res. 2007;51:746–51 [DOI] [PubMed] [Google Scholar]
- 23.Weiss EI, Kozlovsky A, Steinberg D, Lev-Dor R, Bar Ness Greenstein R, Feldman M, Sharon N, Ofek I. A high molecular mass cranberry constituent reduces mutans streptococci level in saliva and inhibits in vitro adhesion to hydroxyapatite. FEMS Microbiol Lett. 2004;232:89–92 [DOI] [PubMed] [Google Scholar]
- 24.Basu A, Betts NM, Ortiz J, Simmons B, Wu M, Lyons TJ. Low-energy cranberry juice decreases lipid oxidation and increases plasma antioxidant capacity in women with metabolic syndrome. Nutr Res. 2011;31:190–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vasileiou I, Katsargyris A, Theocharis S, Giaginis C. Current clinical status on the preventive effects of cranberry consumption against urinary tract infections. Nutr Res. 2013;33:595–607 [DOI] [PubMed] [Google Scholar]
- 26.Pappas E, Schaich KM. Phytochemicals of cranberries and cranberry products: characterization, potential health effects, and processing stability. Crit Rev Food Sci Nutr. 2009;49:741–81 [DOI] [PubMed] [Google Scholar]
- 27.White BL, Howard LR, Prior RL. Impact of different stages of juice processing on the anthocyanin, flavonol, and procyanidin contents of cranberries. J Agric Food Chem. 2011;59:4692–8 [DOI] [PubMed] [Google Scholar]
- 28.Gu L, Kelm MA, Hammerstone JF, Beecher G, Holden J, Haytowitz D, Gebhardt S, Prior RL. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J Nutr. 2004;134:613–7 [DOI] [PubMed] [Google Scholar]
- 29.Foo LY, Lu YR, Howell AB, Vorsa N. The structure of cranberry proanthocyanidins which inhibit adherence of uropathogenic P-fimbriated Escherichia coli in vitro. Phytochemistry. 2000;54:173–81 [DOI] [PubMed] [Google Scholar]
- 30.Feliciano RP, Krueger CG, Shanmuganayagam D, Vestling MM, Reed JD. Deconvolution of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry isotope patterns to determine ratios of A-type to B-type interflavan bonds in cranberry proanthocyanidins. Food Chem. 2012;135:1485–93 [DOI] [PubMed] [Google Scholar]
- 31.Howell AB, Reed JD, Krueger CG, Winterbottom R, Cunningham DG, Leahy M. A-type cranberry proanthocyanidins and uropathogenic bacterial anti-adhesion activity. Phytochemistry. 2005;66:2281–91 [DOI] [PubMed] [Google Scholar]
- 32.Jungfer E, Zimmermann BF, Ruttkat A, Galensa R. Comparing procyanidins in selected vaccinium species by UHPLC-MS(2) with regard to authenticity and health effects. J Agric Food Chem. 2012;60:9688–96 [DOI] [PubMed] [Google Scholar]
- 33.USDA database for proanthocyanidin content of selected foods [database on the Internet]. Beltsville (MD): Nutrient Data Laboratory, Agricultural Research Service, USDA (in collaboration with The Arkansas Children’s Nutrition Center; ARS; USDA; Mars, Inc.; and Ocean Spray Cranberries, Inc.); 2004. [cited 2013 May 28]. Available from: http://www.ars.usda.gov/Services/docs.htm?docid=5843
- 34.Grace MH, Massey AR, Mbeunkui F, Yousef GG, Lila MA. Comparison of health-relevant flavonoids in commonly consumed cranberry products. J Food Sci. 2012;77:H176–83 [DOI] [PubMed] [Google Scholar]
- 35.Reed JD, Krueger CG, Vestling MM. MALDI-TOF mass spectrometry of oligomeric food polyphenols. Phytochemistry. 2005;66:2248–63 [DOI] [PubMed] [Google Scholar]
- 36.Saura-Calixto F. Concept and health-related properties of nonextractable polyphenols: the missing dietary polyphenols. J Agric Food Chem. 2012;60:11195–200 [DOI] [PubMed] [Google Scholar]
- 37.Saura-Calixto F, Serrano J, Goñi I. Intake and bioaccessibility of total polyphenols in a whole diet. Food Chem. 2007;101:492–501 [Google Scholar]
- 38.Pérez-Jiménez J, Fezeu L, Touvier M, Arnault N, Manach C, Hercberg S, Galan P, Scalbert A. Dietary intake of 337 polyphenols in French adults. Am J Clin Nutr. 2011;93:1220–8 [DOI] [PubMed] [Google Scholar]
- 39.Tarascou I, Mazauric JP, Meudec E, Souquet JM, Cunningham D, Nojeim S, Cheynier V, Fulcrand H. Characterisation of genuine and derived cranberry proanthocyanidins by LC-ESI-MS. Food Chem. 2011;128:802–10 [Google Scholar]
- 40.Wu X, Prior RL. Systematic identification and characterization of anthocyanins by HPLC-ESI-MS/MS in common foods in the United States: fruits and berries. J Agric Food Chem. 2005;53:2589–99 [DOI] [PubMed] [Google Scholar]
- 41.Côté J, Caillet S, Doyon G, Sylvain JF, Lacroix M. Analyzing cranberry bioactive compounds. Crit Rev Food Sci Nutr. 2010;50:872–88 [DOI] [PubMed] [Google Scholar]
- 42.Vvedenskaya IO, Vorsa N. Flavonoid composition over fruit development and maturation in American cranberry, Vaccinium macrocarpon Ait. Plant Sci. 2004;167:1043–54 [Google Scholar]
- 43.Celik H, Ozgen M, Serce S, Kaya C. Phytochemical accumulation and antioxidant capacity at four maturity stages of cranberry fruit. Sci Hortic (Amsterdam). 2008;117:345–8 [Google Scholar]
- 44.Brown PN, Murch SJ, Shipley P. Phytochemical Diversity of Cranberry (Vaccinium macrocarpon Aiton) Cultivars by Anthocyanin Determination and Metabolomic Profiling with Chemometric Analysis. J Agric Food Chem 2012;60:261–71 [DOI] [PubMed] [Google Scholar]
- 45.Wu X, Beecher GR, Holden JM, Haytowitz DB, Gebhardt SE, Prior RL. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J Agric Food Chem. 2006;54:4069–75 [DOI] [PubMed] [Google Scholar]
- 46.Crozier A, Del Rio D, Clifford MN. Bioavailability of dietary flavonoids and phenolic compounds. Mol Aspects Med. 2010;31:446–67 [DOI] [PubMed] [Google Scholar]
- 47.Czank C, Cassidy A, Zhang Q, Morrison DJ, Preston T, Kroon PA, Botting NP, Kay CD. Human metabolism and excretion of the anthocyanin, cyanidin-3-glucoside: a 13C- tracer study. Am J Clin Nutr. 2013;97:995–1003 [DOI] [PubMed] [Google Scholar]
- 48.Zuo Y, Wang C, Zhan J. Separation, characterization, and quantitation of benzoic and phenolic antioxidants in American cranberry fruit by GC-MS. J Agric Food Chem. 2002;50:3789–94 [DOI] [PubMed] [Google Scholar]
- 49.Wang C, Zuo Y. Ultrasound-assisted hydrolysis and gas chromatography-mass spectrometric determination of phenolic compounds in cranberry products. Food Chem. 2011;128:562–8 [DOI] [PubMed] [Google Scholar]
- 50.Zhang K, Zuo Y. GC-MS determination of flavonoids and phenolic and benzoic acids in human plasma after consumption of cranberry juice. J Agric Food Chem. 2004;52:222–7 [DOI] [PubMed] [Google Scholar]
- 51.Kondo M, MacKinnon SL, Craft CC, Matchett MD, Hurta RA, Neto CC. Ursolic acid and its esters: occurrence in cranberries and other Vaccinium fruit and effects on matrix metalloproteinase activity in DU145 prostate tumor cells. J Sci Food Agric. 2011;91:789–96 [DOI] [PubMed] [Google Scholar]
- 52.Ikeda Y, Murakami A, Ohigashi H. Ursolic acid: an anti- and pro-inflammatory triterpenoid. Mol Nutr Food Res. 2008;52:26–42 [DOI] [PubMed] [Google Scholar]
- 53.Turner A, Chen SN, Nikolic D, van Breemen R, Farnsworth NR, Pauli GF. Coumaroyl iridoids and a depside from cranberry (Vaccinium macrocarpon). J Nat Prod. 2007;70:253–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mikulic-Petkovsek M, Slatnar A, Stampar F, Veberic R. HPLC-MSn identification and quantification of flavonol glycosides in 28 wild and cultivated berry species. Food Chem. 2012;135:2138–46 [DOI] [PubMed] [Google Scholar]
- 55.Neveu V., Pérez -Jiménez J, Vos F, Crespy V, du Chaffaut L, Mennen L, Knox C, Eisner R, Cruz J, Wishart D, Scalbert A. Phenol-Explorer: an online comprehensive database on polyphenol contents in foods [database on the Internet]. Paris: French National Institute for Agricultural Research (INRA); 2010. [cited 19 September 2013]. Available from: http://www.phenol-explorer.eu/ [DOI] [PMC free article] [PubMed]
- 56.Harnly JM, Doherty RF, Beecher GR, Holden JM, Haytowitz DB, Bhagwat S, Gebhardt S. Flavonoid content of US fruits, vegetables, and nuts. J Agric Food Chem. 2006;54:9966–77 [DOI] [PubMed] [Google Scholar]
- 57.Häkkinen SH, Karenlampi SO, Heinonen IM, Mykkänen HM, Törrönen AR. Content of the flavonols quercetin, myricetin, and kaempferol in 25 edible berries. J Agric Food Chem. 1999;47:2274–9 [DOI] [PubMed] [Google Scholar]
- 58.Keough GR. Massachusetts cranberries [Internet]. Concord (NH): New England Field Office, National Agricultural Statistics Service, USDA; 2013. [cited 19 September 2013]. Available from: http://www.nass.usda.gov/Statistics_by_State/New_England_includes/Publications/jancran.pdf
- 59.Moco S, Bino RJ, Vorst O, Verhoeven HA, de Groot J, van Beek TA, Vervoort J, de Vos CH. A liquid chromatography-mass spectrometry-based metabolome database for tomato. Plant Physiol. 2006;141:1205–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iijima Y, Nakamura Y, Ogata Y, Tanaka K, Sakurai N, Suda K, Suzuki T, Suzuki H, Okazaki K, Kitayama M, et al. Metabolite annotations based on the integration of mass spectral information. Plant J. 2008;54:949–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pallett A, Hand K. Complicated urinary tract infections: practical solutions for the treatment of multiresistant Gram-negative bacteria. J Antimicrob Chemother. 2010;65:iii25–33 [DOI] [PubMed] [Google Scholar]
- 62.Kontiokari T, Laitinen J, Järvi L, Pokka T, Sundqvist K, Uhari M. Dietary factors protecting women from urinary tract infection. Am J Clin Nutr. 2003;77:600–4 [DOI] [PubMed] [Google Scholar]
- 63.Kontiokari T, Nuutinen M, Uhari M. Dietary factors affecting susceptibility to urinary tract infection. Pediatr Nephrol. 2004;19:378–83 [DOI] [PubMed] [Google Scholar]
- 64.Blatherwick NR, Long ML. Studies on urinary acidity: II. The increased acidity produced by eating prunes and cranberries. J Biol Chem. 1923;57:815–8 [Google Scholar]
- 65.Wollenweber E. Occurrence of flavonoid aglycones in medicinal plants. Prog Clin Biol Res. 1988;280:45–55 [PubMed] [Google Scholar]
- 66.Ohno T, Kita M, Yamaoka Y, Imamura S, Yananoto T, Mitsufuji S, Kodama T, Kashima K, Imanishi J. Antimicrobial activity of essential oils against Helicobacter pylori. Helicobacter. 2003;8:207–15 [DOI] [PubMed] [Google Scholar]
- 67.Howell AB, Vorsa N, Der Marderosian A, Foo LY. Inhibition of the adherence of P-fimbriated Escherichia coli to uroepithelial-cell surfaces by proanthocyanidin extracts from cranberries. N Engl J Med. 1998;339:1085–6 [DOI] [PubMed] [Google Scholar]
- 68.Howell AB, Botto H, Combescure C, Blanc-Potard AB, Gausa L, Matsumoto T, Tenke P, Sotto A, Lavigne JP. Dosage effect on uropathogenic Escherichia coli anti-adhesion activity in urine following consumption of cranberry powder standardized for proanthocyanidin content: a multicentric randomized double blind study. BMC Infect Dis. 2010;10:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tao Y, Pinzón-Arango PA, Howell AB, Camesano TA. Oral consumption of cranberry juice cocktail inhibits molecular-scale adhesion of clinical uropathogenic Escherichia coli. J Med Food. 2011;14:739–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu Y, Black MA, Caron L, Camesano TA. Role of cranberry juice on molecular- scale surface characteristics and adhesion behavior of Escherichia coli. Biotechnol Bioeng. 2006;93:297–305 [DOI] [PubMed] [Google Scholar]
- 71.Ahuja S, Kaack B, Roberts JA. Loss of fimbrial adhesion with the addition of Vaccinium macrocarpon to the growth medium of P-fimbriated Escherichia coli. J Urol. 1998;159:559–62 [DOI] [PubMed] [Google Scholar]
- 72.Krueger CG, Reed JD, Feliciano RP, Howell AB. Quantifying and characterizing proanthocyanidins in cranberries in relation to urinary tract health. Anal Bioanal Chem. 2013;405:4385–95 [DOI] [PubMed] [Google Scholar]
- 73.Avorn J, Monane M, Gurwitz JH, Glynn RJ, Choodnovskiy I, Lipsotz LA. Reduction of bacteriuria and pyuria after ingestion of cranberry juice. JAMA. 1994;271:751–4 [DOI] [PubMed] [Google Scholar]
- 74.McMurdo ME, Bissett LY, Price RJ, Phillips G, Crombie IK. Does ingestion of cranberry juice reduce symptomatic urinary tract infections in older people in hospital? A double-blind, placebo-controlled trial. Age Ageing. 2005;34:256–61 [DOI] [PubMed] [Google Scholar]
- 75.Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2012;10:CD001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moen DV. Observations on the effectiveness of cranberry juice in urinary infections. Wis Med J. 1962;61:282–3 [PubMed] [Google Scholar]
- 77.Walker EB, Barney DP, Mickelsen JN, Walton RJ, Mickelsen RA., Jr Cranberry concentrate: UTI prophylaxis. J Fam Pract. 1997;45:167–8 [PubMed] [Google Scholar]
- 78.Barbosa-Cesnik C, Brown MB, Buxton M, Zhang L, DeBusscher J, Foxman B. Cranberry juice fails to prevent recurrent urinary tract infection: results from a randomized placebo-controlled trial. Clin Infect Dis. 2011;52:23–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stothers L. A randomized trial to evaluate effectiveness and cost effectiveness of naturopathic cranberry products as prophylaxis against urinary tract infection in women. Can J Urol. 2002;9:1558–62 [PubMed] [Google Scholar]
- 80.Stapleton AE, Dziura J, Hooton TM, Cox ME, Yarova-Yarovaya Y, Chen S, Gupta K. Recurrent urinary tract infection and urinary Escherichia coli in women ingesting cranberry juice daily: a randomized controlled trial. Mayo Clin Proc. 2012;87:143–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kontiokari T, Sundqvist K, Nuutinen M, Pokka T, Koskela M, Uhari M. Randomized trial of cranberry-lingonberry juice and Lactobacillus GG drink for the prevention of urinary tract infections in women. BMJ. 2001;322:1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Foxman B, Geiger AM, Palin K, Gillespie B, Koopman JS. First-time urinary tract infection and sexual behavior. Epidemiology. 1995;6:162–8 [DOI] [PubMed] [Google Scholar]
- 83.Ferrara P, Romaniello L, Vitelli O, Gatto A, Serva M, Cataldi L. Cranberry juice for the prevention of recurrent urinary tract infections: a randomized controlled trial in children. Scand J Urol Nephrol. 2009;43:369–72 [DOI] [PubMed] [Google Scholar]
- 84.Foda MM, Middlebrook PF, Gatfield CT, Potvin G, Wells G, Schillinger JF. Efficacy of cranberry in prevention of urinary tract infection in a susceptible pediatric population. Can J Urol. 1995;2:98–102 [PubMed] [Google Scholar]
- 85.Salo J, Uhari M, Helminen M, Korppi M, Nieminen T, Pokka T, Kontiokari T. Cranberry juice for the prevention of recurrences of urinary tract infections in children: a randomized placebo-controlled trail. Clin Infect Dis. 2012;54:340–6 [DOI] [PubMed] [Google Scholar]
- 86.Afshar K, Stothers L, Scott H, MacNeily AE. Cranberry juice for prevention of pediatric urinary tract infection: a randomized controlled trial. J Urol. 2012;188:1584–7 [DOI] [PubMed] [Google Scholar]
- 87.Serrano J, Puupponen-Pimiä R, Dauer A, Aura A-M, Saura-Calixto F. Tannins: current knowledge of food sources, intake, bioavailability and biological effects. Mol Nutr Food Res. 2009;53:S310–29 [DOI] [PubMed] [Google Scholar]
- 88.Schmidt BM, Howell AB, McEniry B, Knight CT, Seigler D, Erdman JW, Jr, Lila MA. Effective separation of potent antiproliferation and antiadhesion components from wild blueberry (Vaccinium angustifolium Ait.) fruits. J Agric Food Chem. 2004;52:6433–42 [DOI] [PubMed] [Google Scholar]
- 89.Kimura H, Ogawa S, Akihiro T, Yokota K. Structural analysis of A-type or B-type highly polymeric proanthocyanidins by thiolytic degradation and the implication in their inhibitory effects on pancreatic lipase. J Chromatogr A. 2011;1218:7704–12 [DOI] [PubMed] [Google Scholar]
- 90.Kylli P, Nohynek L, Puupponen-Pimiä R, Westerlund-Wikström B, Leppänen T, Welling J, Moilanen E, Heinonen M. Lingonberry (Vaccinium vitis-idaea) and European cranberry (Vaccinium microcarpon) proanthocyanidins: isolation, identification, and bioactivities. J Agric Food Chem. 2011;59:3373–84 [DOI] [PubMed] [Google Scholar]
- 91.Ofek I, Goldhar J, Zafriri D, Lis H, Adar R, Sharon N. Anti-Escherichia coli adhesin activity of cranberry and blueberry juices. N Engl J Med. 1991;324:1599. [DOI] [PubMed] [Google Scholar]
- 92.Ofek I, Goldhar J, Sharon N. Anti-Escherichia coli adhesion activity of cranberry and blueberry juices. Adv Exp Med Biol. 1996;408:179–83 [DOI] [PubMed] [Google Scholar]
- 93.Kalgaonkar S, Gross HB, Yokoyama W, Keen CL. Effects of a flavonol-rich diet on select cardiovascular parameters in a Golden Syrian hamster model. J Med Food. 2010;13:108–15 [DOI] [PubMed] [Google Scholar]
- 94.Yung LM, Tian XY, Wong WT, Leung FP, Yung LH, Chen ZY, Lau CW, Vanhoutte PM, Yao X, Huang Y. Chronic cranberry juice consumption restores cholesterol profiles and improves endothelial function in ovariectomized rats. Eur J Nutr. 2013;52:1145–55 [DOI] [PubMed] [Google Scholar]
- 95.Reed J. Cranberry flavonoids, atherosclerosis, and cardiovascular health. Crit Rev Food Sci Nutr. 2002;42 Suppl:301–16 [DOI] [PubMed] [Google Scholar]
- 96.Zhu Y, Ling W, Guo H, Song F, Ye Q, Zou T, Li D, Zhang Y, Li G, Xiao Y, et al. Anti-inflammatory effect of purified dietary anthocyanin in adults with hypercholesterolemia: a randomized controlled trial. Nutr Metab Cardiovasc Dis. [DOI] [PubMed] [Google Scholar]
- 97.Qin Y, Xia M, Ma J, Hao Y, Liu J, Mou H, Cao L, Ling W. Anthocyanin supplementation improves serum LDL- and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am J Clin Nutr. 2009;90:485–92 [DOI] [PubMed] [Google Scholar]
- 98.Chu YF, Liu RH. Cranberries inhibit LDL oxidation and induce LDL receptor expression in hepatocytes. Life Sci. 2005;77:1892–901 [DOI] [PubMed] [Google Scholar]
- 99.Tall AR. CETP inhibitors to increase HDL cholesterol levels. N Engl J Med. 2007;356:1364–6 [DOI] [PubMed] [Google Scholar]
- 100.Maher MA, Mataczynski H, Stefaniak HM, Wilson T. Cranberry juice induces nitric oxide-dependent vasodilation in vitro and its infusion transiently reduces blood pressure in anesthetized rats. J Med Food. 2000;3:141–7 [DOI] [PubMed] [Google Scholar]
- 101.Apostolidis E, Kwon YI, Shetty K. Potential of cranberry-based herbal synergies for diabetes and hypertension management. Asia Pac J Clin Nutr. 2006;15:433–41 [PubMed] [Google Scholar]
- 102.Hassellund SS, Flaa A, Sandvik L, Kjeldsen SE, Rostrup M. Effects of anthocyanins on blood pressure and stress reactivity: a double-blind randomized placebo-controlled crossover study. J Hum Hypertens. 2012;26:396–404 [DOI] [PubMed] [Google Scholar]
- 103.Khanal RC, Rogers TJ, Wilkes SE, Howard LR, Prior RL. Effects of dietary consumption of cranberry powder on metabolic parameters in growing rats fed high fructose diets. Food Funct. 2010;1:116–23 [DOI] [PubMed] [Google Scholar]
- 104.Shabrova EV, Tarnopolsky O, Singh AP, Plutzky J, Vorsa N, Quadro L. Insights into the molecular mechanisms of the anti-atherogenic actions of flavonoids in normal and obese mice. PLoS ONE. 2011;6:e24634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chambers BK, Camire ME. Can cranberry supplementation benefit adults with type 2 diabetes? Diabetes Care. 2003;26:2695–6 [DOI] [PubMed] [Google Scholar]
- 106.Stocker R, Keaney JF., Jr Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–478 [DOI] [PubMed] [Google Scholar]
- 107.Münzel T, Keaney JF., Jr Are ACE-inhibitors a "magic bullet" against oxidative stress? Circulation. 2001;104:1571–4 [DOI] [PubMed] [Google Scholar]
- 108.Basu A, Rhone M, Lyons TJ. Berries: emerging impact on cardiovascular health. Nutr Rev. 2010;68:168–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hollman PC, Cassidy A, Comte B, Heinonen M, Richelle M, Richling E, Serafini M, Scalbert A, Sies H, Vidry S. The biological relevance of direct antioxidant effects of polyphenols for cardiovascular health in humans is not established. J Nutr. 2011;141 Suppl:989S–1009S [DOI] [PubMed] [Google Scholar]
- 110.Ruel G, Pomerleau S, Couture P, Lemieux S, Lamarche B, Couillard C. Low-calorie cranberry juice supplementation reduces plasma oxidized LDL and cell adhesion molecule concentrations in men. Br J Nutr. 2008;99:352–9 [DOI] [PubMed] [Google Scholar]
- 111.Mora S, Glynn RJ, Hsia J, MacFadyen JG, Genest J, Ridker PM. Statins for the primary prevention of cardiovascular events in women with elevated high-sensitivity C-reactive protein or dyslipidemia: results from the Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) and meta-analysis of women from primary prevention trials. Circulation. 2010;121:1069–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bodet C, Chandad F, Grenier D. Anti-inflammatory activity of a high-molecular-weight cranberry fraction on macrophages stimulated by lipopolysaccharides from periodontopathogens. J Dent Res. 2006;85:235–9 [DOI] [PubMed] [Google Scholar]
- 113.Huang Y, Nikolic D, Pendland S, Doyle BJ, Locklear TD, Mahady GB. Effects of cranberry extracts and ursolic acid derivatives on P-fimbriated Escherichia coli, COX-2 activity, pro-inflammatory cytokine release and the NF-kappa beta transcriptional response in vitro. Pharm Biol. 2009;47:18–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bodet C, Chandad F, Grenier D. Cranberry components inhibit interleukin-6, interleukin-8, and prostaglandin E production by lipopolysaccharide-activated gingival fibroblasts. Eur J Oral Sci. 2007;115:64–70 [DOI] [PubMed] [Google Scholar]
- 115.Youdim KA, McDonald J, Kalt W, Joseph JA. Potential role of dietary flavonoids in reducing microvascular endothelium vulnerability to oxidative and inflammatory insults. J Nutr Biochem. 2002;13:282–8 [DOI] [PubMed] [Google Scholar]
- 116.Cimino F, Speciale A, Anwar S, Canali R, Ricciardi E, Virgili F, Trombetta D, Saija A. Anthocyanins protect human endothelial cells from mild hyperoxia damage through modulation of Nrf2 pathway. Genes Nutr. 2013;8:391–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–60 [DOI] [PubMed] [Google Scholar]
- 118.Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Luscher TF, Shechter M, Taddei S, et al. The assessment of endothelial function: from research into clinical practice. Circulation. 2012;126:753–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tulio AZ, Jr, Chang C, Edirisinghe I, White KD, Jablonski JE, Banaszewski K, Kangath A, Tadapaneni RK, Burton-Freeman B, Jackson LS. Berry fruits modulated endothelial cell migration and angiogenesis via phosphoinositide-3 kinase/protein kinase B pathway in vitro in endothelial cells. J Agric Food Chem. 2012;60:5803–12 [DOI] [PubMed] [Google Scholar]
- 120.Paixão J, Dinis TC, Almeida LM. Malvidin-3-glucoside protects endothelial cells up-regulating endothelial NO synthase and inhibiting peroxynitrite-induced NF-kB activation. Chem Biol Interact. 2012;199:192–200 [DOI] [PubMed] [Google Scholar]
- 121.Milbury PE, Vita JA, Blumberg JB. Anthocyanins are bioavailable in humans following an acute dose of cranberry juice. J Nutr. 2010;140:1099–104 [DOI] [PubMed] [Google Scholar]
- 122.Widlansky ME, Hamburg NM, Anter E, Holbrook M, Kahn DF, Elliott JG, Keaney JF, Jr, Vita JA. Acute EGCG supplementation reverses endothelial dysfunction in patients with coronary artery disease. J Am Coll Nutr. 2007;26:95–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sies H. Polyphenols and health: update and perspectives. Arch Biochem Biophys. 2010;501:2–5 [DOI] [PubMed] [Google Scholar]
- 124.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308:875–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mitchell GF. Arterial stiffness and wave reflection: biomarkers of cardiovascular risk. Artery Res. 2009;3:56–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ruel G, Lapointe A, Pomerleau S, Couture P, Lemieux S, Lamarche B, Couillard C. Evidence that cranberry juice may improve augmentation index in overweight men. Nutr Res. 2013;33:41–9 [DOI] [PubMed] [Google Scholar]
- 128.Deka A, Vita JA. Tea and cardiovascular disease. Pharmacol Res. 2011;64:136–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Freedman JE, Parker C, III, Li L, Perlman JA, Frei B, Ivanov V, Deak LR, Iafrati MD, Folts JD. Select flavonoids and whole juice from purple grapes inhibit platelet function and enhance nitric oxide release. Circulation. 2001;103:2792–8 [DOI] [PubMed] [Google Scholar]
- 130.Yang Y, Shi Z, Reheman A, Jin JW, Li C, Wang Y, Andrews MC, Chen P, Zhu G, Ling W, et al. Plant food delphinidin-3-glucoside significantly inhibits platelet activation and thrombosis: novel protective roles against cardiovascular diseases. PLoS ONE. 2012;7:e37323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wilson T, Meyers SL, Singh AP, Limburg PJ, Vorsa N. Favorable glycemic response of type 2 diabetics to low-calorie cranberry juice. J Food Sci. 2008;73:H241–5 [DOI] [PubMed] [Google Scholar]
- 132.Wilson T, Singh AP, Vorsa N, Goettl CD, Kittleson KM, Roe CM, Kastello GM, Ragsdale FR. Human glycemic response and phenolic content of unsweetened cranberry juice. J Med Food. 2008;11:46–54 [DOI] [PubMed] [Google Scholar]
- 133.Shidfar F, Heydari I, Hajimiresmaiel SJ, Hosseini S, Shidfar S, Amiri F. The effects of cranberry juice on serum glucose, apoB, apoA-I, Lp(a), and paraoxonase-1 activity in type 2 diabetic male patients. J Res Med Sci. 2012;17:355–60 [PMC free article] [PubMed] [Google Scholar]
- 134.Ruel G, Pomerleau S, Couture P, Lemieux S, Lamarche B, Couillard C. Plasma matrix metalloproteinase (MMP)-9 levels are reduced following low-calorie cranberry juice supplementation in men. J Am Coll Nutr. 2009;28:694–701 [DOI] [PubMed] [Google Scholar]
- 135.Duthie GG, Kyle JA, Jenkinson AM, Duthie SJ, Baxter GJ, Paterson JR. Increased salicylate concentrations in urine of human volunteers after consumption of cranberry juice. J Agric Food Chem. 2005;53:2897–900 [DOI] [PubMed] [Google Scholar]
- 136.Mink PJ, Scrafford CG, Barraj LM, Harnack L, Hong CP, Nettleton JA, Jacobs DR., Jr Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. Am J Clin Nutr. 2007;85:895–909 [DOI] [PubMed] [Google Scholar]
- 137.McCullough ML, Peterson JJ, Patel R, Jacques PF, Shah R, Dwyer JT. Flavonoid intake and cardiovascular disease mortality in a prospective cohort of US adults. Am J Clin Nutr. 2012;95:454–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cassidy A, Mukamal KJ, Liu L, Franz M, Eliassen AH, Rimm EB. High anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation. 2013;127:188–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mursu J, Voutilainen S, Nurmi T, Tuomainen TP, Kurl S, Salonen JT. Flavonoid intake and the risk of ischaemic stroke and CVD mortality in middle-aged Finnish men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Br J Nutr. 2008;100:890–5 [DOI] [PubMed] [Google Scholar]
- 140.Cassidy A, Rimm EB, O’Reilly EJ, Logroscino G, Kay C, Chiuve SE, Rexrode KM. Dietary flavonoids and risk of stroke in women. Stroke. 2012;43:946–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cassidy A, O'Reilly EJ, Kay C, Sampson L, Franz M, Forman J, Curhan G, Rimm EB. Habitual intake of flavonoid subclasses and incident hypertension in adults. Am J Clin Nutr. 2011;93:338–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jennings A, Welch AA, Fairweather-Tait SJ, Kay C, Minihane AM, Chowienczyk P, Jiang B, Cecelja M, Spector T, Macgregor A, et al. Higher anthocyanin intake is associated with lower arterial stiffness and central blood pressure in women. Am J Clin Nutr. 2012;96:781–8 [DOI] [PubMed] [Google Scholar]
- 143.Huxley RR, Neil HA. The relation between dietary flavonol intake and coronary heart disease mortality: a meta-analysis of prospective cohort studies. Eur J Clin Nutr. 2003;57:904–8 [DOI] [PubMed] [Google Scholar]
- 144.Wang ZM, Nie ZL, Zhou B, Lian XQ, Zhao H, Gao W, Wang YS, Jia EZ, Wang LS, Yang ZJ. Flavonols intake and the risk of coronary heart disease: a meta-analysis of cohort studies. Atherosclerosis. 2012;222:270–3 [DOI] [PubMed] [Google Scholar]
- 145.Hollman PC, Geelen A, Kromhout D. Dietary flavonol intake may lower stroke risk in men and women. J Nutr. 2010;140:600–4 [DOI] [PubMed] [Google Scholar]
- 146.Mursu J, Nurmi T, Tuomainen TP, Ruusunen A, Salonen JT, Voutilainen S. The intake of flavonoids and carotid atherosclerosis: the Kuopio Ischaemic Heart Disease Risk Factor Study. Br J Nutr. 2007;98:814–8 [DOI] [PubMed] [Google Scholar]
- 147.Chun OK, Chung SJ, Claycombe KJ, Song WO. Serum C-reactive protein concentrations are inversely associated with dietary flavonoid intake in US adults. J Nutr. 2008;138:753–60 [DOI] [PubMed] [Google Scholar]
- 148.Landberg R, Sun Q, Rimm EB, Cassidy A, Scalbert A, Mantzoros CS, Hu FB, van Dam RM. Selected dietary flavonoids are associated with markers of inflammation and endothelial dysfunction in U.S. women. J Nutr. 2011;141:618–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Arts ICW, Jacobs DR, Jr, Harnack LJ, Gross M, Folsom AR. Dietary catechins in relation to coronary heart disease death among postmenopausal women. Epidemiology. 2001;12:668–75 [DOI] [PubMed] [Google Scholar]
- 150.Arts ICW, Hollman PCH, Feskens EJM, Bueno de Mesquita HB, Kromhout D. Catechin intake might explain the inverse relation between tea consumption and ischemic heart disease: the Zutphen Elderly Study. Am J Clin Nutr. 2001;74:227–32 [DOI] [PubMed] [Google Scholar]
- 151.Bhagwat SA, Haytowitz DB, Holden JM. USDA database for the flavonoid content of selected foods, release 3 [database on the Internet]. Beltsville (MD): Agricultural Research Service, USDA; 2011. [cited 2013 May 28]. Available from: http://www.ars.usda.gov/Services/docs.htm?docid=6231
- 152.Crozier A, Jaganath IB, Clifford MN. Dietary phenolics: chemistry, bioavailability and effects on health. Nat Prod Rep. 2009;26:1001–43 [DOI] [PubMed] [Google Scholar]
- 153.Wang Y, Chung SJ, Song WO, Chun OK. Estimation of daily proanthocyanidin intake and major food sources in the US Diet. J Nutr. 2011;141:447–52 [DOI] [PubMed] [Google Scholar]
- 154.Zamora-Ros R, Knaze V, Luján-Barroso L, Romieu I, Scalbert A, Slimani N, Hjartåker A, Engeset D, Skeie G, Overvad K, et al. Differences in dietary intakes, food sources and determinants of total flavonoids between Mediterranean and non-Mediterranean countries participating in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Br J Nutr. 2013;109:1498–507 [DOI] [PubMed] [Google Scholar]
- 155.Knaze V, Zamora-Ros R, Luján-Barroso, Romieu I, Scalbert A, Slimani N, Riboli E, van Rossum CT, Bueno-de-Mesquita HB, Trichopoulou A, et al. Intake estimation of total and individual flavan-3-ols, proanthocyanidins and theaflavins, their food sources and determinants in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Br J Nutr. 2012;108:1095–108 [DOI] [PubMed] [Google Scholar]
- 156.Mullen W, Marks SC, Crozier A. Evaluation of phenolic compounds in commercial fruit juices and fruit drinks. J Agric Food Chem. 2007;55:3148–57 [DOI] [PubMed] [Google Scholar]