Abstract
Purpose
Transplantation of ex vivo expanded limbal epithelial progenitor cells (LEPCs) on epithelially denuded amniotic membrane (dAM) in supplemented hormonal epithelial medium (SHEM) is an alternative solution for treating corneal blindness due to limbal stem cell (SC) deficiency. Because the phenotype of limbal niche cells (NCs) is preserved better in serum-free modified embryonic stem cell (ESC) medium (MESCM) than SHEM, we question whether the aforementioned expansion protocol can be further optimized by maintaining limbal NCs using MESCM.
Methods
Collagenase-isolated limbal clusters were cultured on dAM in SHEM or MESCM for 8 to 10 days. Epithelial outgrowth sheets removed by dispase were subjected to real-time quantitative polymerase chain reaction (qPCR) and immunostaining for expression of corneal epithelial markers (p63α, pax6, and K12) and NC markers (FLK-1, CD34, CD31, PDGFR-B, and α-SMA). A total of 1000 single cells were seeded on 6-well dish containing 3T3 feeder layers for 12 to 14 days before rhodamine B staining.
Results
Epithelial outgrowth in SHEM showed a significant loss of corneal SC and ESC markers when compared with freshly collagenase-isolated limbal clusters. Although the epithelial outgrowth was slower in MESCM, epithelial cell size was consistently smaller than that found in SHEM. Furthermore, MESCM maintained a significantly higher percentage of PCK−/ Vim+ cells and exhibited a significant upregulation of NC markers and corneal epithelial SC markers (K15, Bmi-1, and Msi-1) than SHEM. Furthermore, the number of purported holoclones was significantly promoted in MESCM than SHEM.
Conclusion
These data collectively suggest that MESCM can be used to replace SHEM to further promote expansion of LEPC by maintaining limbal native NCs.
Translational Relevance
Effective ex vivo expansion of limbal epithelial SC is a first and important step toward the success of treating corneal blindness caused by limbal stem cell deficiency and paves the way for future applications in regenerative medicine.
Keywords: cornea, ex vivo expansion, limbus, niche cells, stem cells, stem cell niche
Introduction
Quiescence, self-renewal, and fate decision of stem cells (SCs) are regulated in an anatomically defined niche, which consists of extracellular matrix components and supporting cells termed niche cells (NCs).1 The niche for corneal epithelial SCs is located in the limbal palisade of Vogt.2 Clinically, destructive loss of limbal epithelial SCs or dysfunction of the supporting limbal niche are the two major pathogenic processes leading to limbal SC deficiency that can be found in a number of ocular surface diseases.3 Patients with limbal SC deficiency suffer from severe loss of vision and a poor quality of life. Because limbal SC deficiency is accompanied by conjunctival epithelial ingrowth, vascularization, chronic inflammation, and scarring, these patients are poor candidates for conventional corneal transplantation, of which the therapeutic benefit is restricted by a short-life of transient amplifying cells that have been transplanted.
One alternative solution to treat corneal blindness caused by limbal SC deficiency is transplantation of ex vivo expanded limbal epithelial progenitor cells (LEPC). Because the amniotic membrane contains a basement membrane resembling that from the cornea4 and the conjunctiva5 and such growth factors such as EGF, KGF, HGF, bFGF,6,7 and NGF,8 it has been considered an ideal matrix to support ex vivo expansion of LEPC.9 Indeed, the amniotic membrane together with a serum-containing medium called supplemented hormonal epithelial medium (SHEM) has been used in five of the six protocols that have successfully been used to engineer a surgical graft containing LEPC for treating patients with limbal SC deficiency.10 Nonetheless, none of these protocols have included strategies of isolating and expanding NCs during ex vivo expansion.
We have discovered that collagenase can, but dispase cannot, isolate a cluster of entire basal LEPC, subjacent basement membrane, and a subset of mesenchymal cells negatively expressing pancytokeratin (PCK), but positively expressing vimentin (Vim) from the limbus.11 When freshly isolated, these PCK−/Vim+ cells can be as small as 5 μm in diameter and heterogeneously express embryonic SC (ESC) markers such as Oct4, Sox2, Nanog, Rex1, and SSEA4 as well as other SC markers such as Nestin, N-cadherin, and CD34.11 From collagenase-isolated limbal clusters, a homogeneous population of PCK−/Vim+ cells can be successfully isolated and expanded up to 12 passages on coated Matrigel in a serum-free ESC medium containing bFGF and LIF (termed modified embryonic stem cells medium [MESCM]).12,13 Although during expansion they lose expression of ESC markers,12,13 they regain the expression of ESC markers and other angiogenesis markers such as Flk-1, CD31, CD34, PDGFRβ, α-SMA upon being reseeded in three-dimensional (3D) Matrigel in MESCM.13 In contrast, limbal NCs expanded in SHEM or Dulbecco's modified Eagle's Medium (DMEM)/10% fetal bovine serum (FBS) cannot regain expression of ESC markers upon being reseeded in 3D Matrigel containing MESCM to prevent LEPC from undergoing differentiation in the sphere culture.12 These results collectively allow us to assign them as limbal NCs, of which the phenotype expression of ESC markers is correlated with their supporting role in preventing LEPC from undergoing differentiation.12,13
Indeed, inclusion of NCs from collagenase-isolated clusters has better maintained the phenotype and the clonal expansion of LEPC than dispase-isolated sheet using the conventional ex vivo expansion protocol that is based on AM and cultured in SHEM.11 Herein, we provide experimental evidence further supporting that switching the culture medium from SHEM to MESCM is another strategy that can further help preserve the clonal expansion of LEPC by maintaining the phenotype of limbal NCs.
Materials and Methods
Isolation of Limbal Epithelial Sheets and Clusters
Human corneoscleral rims from donors aged 21 to 68 (42.9 ± 14.9) years were provided by the Florida Lions Eye Bank (Miami, FL) and handled according to the Declaration of Helsinki. Immediately after the central corneal button used for corneal transplantation, they were transferred in Optisol-GS (http://www.bausch.com; Bausch & Lomb, Rochester, NY) and transported at 4°C to the laboratory. All materials used for cell culture are listed as supplemental data (Supplementary Table S1 (116.5KB, doc) ). As reported,11 the rim was first rinsed three times with Hank's Balanced Salt Solution (HBSS) containing gentamicin and amphotericin B. After removal of excessive sclera, conjunctiva, iris, and corneal endothelium, the tissue was cut into 12, one o'clock-hour segments, from which a limbal segment was obtained by incisions made at 1 mm within and beyond the anatomic limbus. An intact epithelial sheet was harvested by digesting each limbal segment with 10 mg/mL dispase containing SHEM14 at 4°C for 16 hours under humidified 5% CO2. In parallel, other limbal segments, without any further trimming off any stromal tissue, were directly digested with 1 mg/mL collagenase A in SHEM or MESCM containing MESCM made of DMEM/F-12 (1:1) supplemented with 10% knockout serum, 5 g/mL insulin, 5 g/mL transferrin, 5ng/mL sodium selenite, 4 ng/mL bFGF, 10 ng/mL hLIF, 50 g/mL gentamicin, and 1.25 g/mL amphotericin B at 37°C for 18 hours under humidified 5% CO2 to generate limbal clusters.
Ex Vivo Expansion on Epithelially-Denuded Amniotic Membrane
Epithelially-denuded amniotic membrane (dAM) was prepared by subjecting cryopreserved AM (Bio-Tissue, Inc., Miami, FL) to incubation with 0.02% EDTA in phosphate buffer solution (PBS) at 37°C for 1 hour to loosen amniotic epithelial cells, followed by gentle mechanical scraping with a toothbrush. dAM was then fastened onto a culture insert as previously reported.15 On each dAM, a freshly collagenase-isolated limbal cluster or dispase-isolated limbal epithelial sheet was manually transferred through a pipette and cultured in SHEM or MESCM. The culture medium was changed every 2 to 3 days. Epithelial outgrowth was monitored under phase contrast microscopy and terminated on day 8 followed crystal violet staining and immunofluorescence staining. The analysis of outgrowths was measured in digitized images by ImageJ (National Institutes of Health, Bethesda, MD) to calculate the surface area.
Clonal Cultures
The clonal culture was performed on 3T3 fibroblast feeder layers, which were prepared by treating 80% subconfluent 3T3 fibroblasts with 4 μg/mL mitomycin C at 37°C for 2 hours in DMEM containing 10% FCS, and then by seeding mitomycin C–treated 3T3 fibroblasts at a density of 2 × 104/cm2. For initiating clonal growth, a total of 1000 single cells derived from outgrowth sheets in SHEM or MESCM on day 7 were seeded in mitomycin C–treated 3T3 6-well plate and cultured in SHEM for 10 days. Clone morphology was categorized as purported holoclone, meroclone, and paraclone based on the morphology, but not secondary cultures described for skin keratinocytes.16 For analysis, all clones were fixed in cold methanol and subjected to staining with either rhodamine B or to immunofluorescence staining.
Immunofluorescence Staining
Single cells suspension from dispase-isolated or collagenase-isolated outgrowth sheets were prepared on slides by cytospin (Cytofuge; StatSpin, Inc., Norwood, MA) at 1000 rpm for 8 minutes at the density of 4.0 × 104 cells per chamber. Slides were air-dried for 5 minutes prior to being fixed with either 100% cold methanol at −20°C or 4% paraformaldehyde at room temperature for 15 minutes. For immunofluorescence staining, cells were permeated with 0.2% Triton X-100 in PBS for 15 to 30 minutes and blocked with 0.2% bovine serum albumin (BSA) in PBS for 1 hour prior to addition of primary antibody overnight. Secondary antibodies were then incubated for 1 hour before image analysis. Isotype-matched non-specific immunoglobulin G (IgG) antibodies were used as controls. Detailed information about primary and secondary antibodies is listed as supplemental data (Supplementary Table S2 (116.5KB, doc) ). Immunofluorescence micrographs were taken by confocal microscopy (LSM 700; Carl Zeiss, Thornhood, NY); cell counting, cell size, and cytolocalization were analyzed using AxioVision software (Carl Zeiss).
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Ex vivo expanded LEPC were isolated from dAM on day 7 by dispase digestion at 37°C for 2 hours for total RNA extraction by RNeasy Mini RNA isolation kit (Qiagen, Valencia, CA) and for preparation of cDNA by reverse-transcription using high capacity cDNA transcription kit (Applied Biosystems, Foster City, CA). Quantitative polymerase chain reaction (qPCR) amplification of different genes was carried out in a 20 μL solution containing cDNA, TaqMan Gene Expression Assay Mix, and universal PCR master Mix (Applied Biosystems) by 7300 real time PCR machine system (Applied Biosystems) according to the manufacturer's description to use the following thermocycler parameters: 10 minutes of initial activation at 95°C, 40 cycles of 15 seconds denaturation at 95°C, and 1 minute annealing and extension at 60°C. All TagMan Gene Expression Assays probes of their sequences are listed as supplemental data (Supplementary Table S3 (116.5KB, doc) ) and performed in triplicate from each donor. The relative gene expression was normalized by an internal control, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and analyzed by the comparative CT method (ΔΔCT).
Statistical Analysis
All assays were performed in triplicate, each with a minimum of three donors. The data are reported as means ± SD. Group means were compared using the appropriate version of Student's unpaired t-test. Test results were reported as two-tailed P values, where P less than 0.05 were considered statistically significant.
Results
Loss of Expression of Limbal Epithelial and Embryonic SC Markers During Conventional Ex Vivo Expansion in SHEM
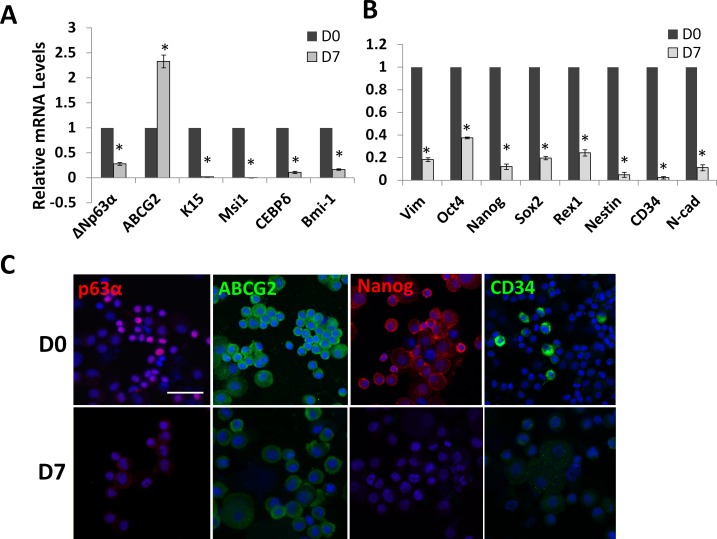
Previously, we have demonstrated that collagenase-isolated limbal clusters failed to express the putative ESC markers if digestion was carried out in SHEM or DMEM/10% FBS.12 Because there was a transient loss of ESC markers by isolated limbal NCs during serial expansion on coated Matrigel in MESCM, but a permanent loss of these markers in SHEM or DMEM/10% FBS,12 we would like to know whether outgrowth from collagenase-isolated clusters on dAM in SHEM, a method we have reported to be superior to dispase-isolated epithelial sheets in maintaining clonal expansion of LEPC,11 could successfully maintain the phenotype of limbal NCs of expressing ESC markers. qPCR analysis of the entire outgrowth after 7 days of culture of collagenase-isolated limbal clusters showed that expressions of markers for corneal progenitors and SC such as ΔNp63α,17 K15,18 Msi1,19 CEBPδ,20 and Bmi-1,21 except ABCG2,22 were significantly downregulated when compare with freshly-isolated limbal clusters (Fig. 1A). Similarly, the expression of ESC markers such as Oct4, Sox2, Nanog, and Rex1 was significantly downregulated (Fig. 1A). Immunostaining of resultant outgrowth after 7 days of culture in SHEM showed weaker expressions of p63α, ABCG2, Nanog, and CD34 in SHEM compared with that of freshly isolated limbal clusters (Fig. 1B). Collectively, these findings suggested that cells expanded on dAM in SHEM had lost expression of markers for both LEPC and limbal NCs.
Figure 1. .
Loss of expression of epithelial and embryonic SC markers during ex vivo expansion in SHEM. When compared with freshly isolated limbal clusters on D0, qPCR analysis of the outgrowth derived from collagenase-isolated limbal clusters cultured on dAM in SHEM for 7 days showed a significant reduction of expression of limbal epithelial SC markers such as ΔNp63α, ABCG2, K15, Msi1, CEBPδ, and Bmi-1, but not ABCG2 (A, n = 3, *P < 0.01). Similarly, expression of all markers expressed by limbal NCs such as Vim+, Oct4, Nanog, Sox2, Rex1, Nestin, CD34, and N-cad was also significantly reduced (B, n = 3, *P < 0.01). Immunostaining of the cytospin preparation showed less expression of p63α (red), ABCG2 (green), Nanog (red), and CD34 (green) staining. Scale bar = 50 μm.
Outgrowth Size in MESCM Is Smaller than That in SHEM
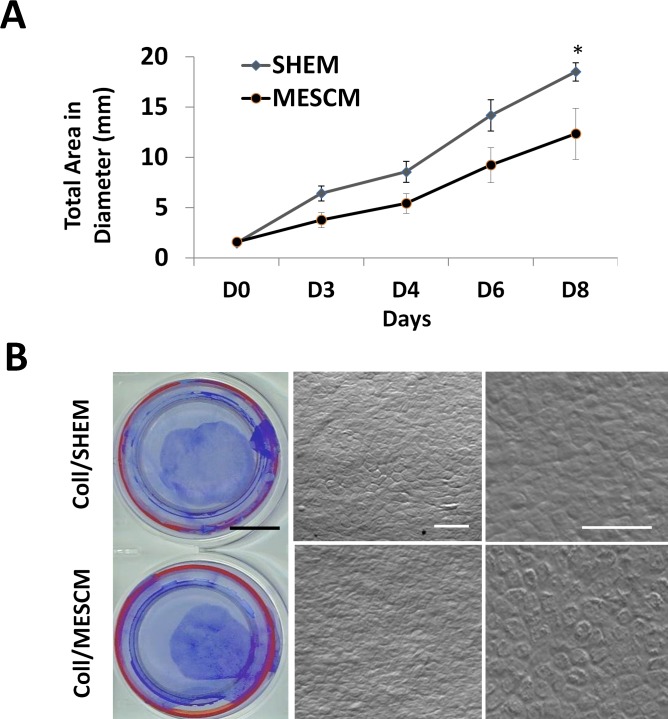
Because loss of ESC markers in SHEM on dAM suggested a potential loss of limbal NCs and because the phenotype of limbal NCs can be better maintained in serum free MESCM, but not in SHEM or DMEM+10% FBS,12 we speculated that MESCM might be a better medium to expand limbal NCs. Collagenase isolated limbal clusters were cultured on dAM in SHEM or MESCM and generated similar circular outgrowth (Fig. 2). The outgrowth generated in both media grew slow on the first 4 days, but exhibited marked expansion from day 5 to 8 (Fig. 2A). Overall, the resultant outgrowth sheets were significantly smaller in MESCM than that in SHEM on day 8 (n = 3, P < 0.01, Fig. 2A) as outlined by crystal violet (Fig. 2B). Phase contrast micrographs revealed that cells derived from MESCM were compact and cuboidal while that derived from SHEM were more irregular (Fig. 2B).
Figure 2. .
Smaller epithelial outgrowth in MESCM than SHEM. Collagenase-isolated clusters (Coll) could expand into outgrowth sheets in SHEM or MESCM on dAM. The growth curve based on the measurement of the digitized images showed a significantly smaller outgrowth size in MESCM than SHEM on day 8 (A, *P < 0.05, n = 3). A representative outgrowth on day 8 was displayed by crystal violet staining (B). The phase contrast micrograph further disclosed small and compact cells in the outgrowth in both SHEM and MESCM (B). Black scale bar = 10 mm; white scale bar = 50 μm.
Limbal Niche Cells are Better Maintained in MESCM than in SHEM
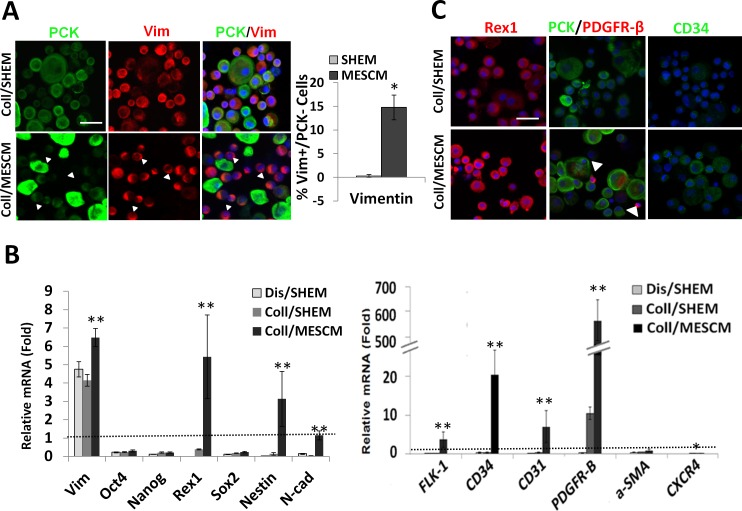
Because cells derived from collagenase outgrowth in MESCM were smaller, compact, and cuboidal, we speculated that MESCM might have better preserved both LEPC and limbal NCs on dAM. To examine this hypothesis, double immunostaining to PCK and Vim was performed using cytospin preparations of single cells derived from the outgrowth cultured in SHEM or MESCM. The results showed that PCK−/Vim+ cells were enriched in the outgrowth cultured in MESCM (Fig. 3A, white arrows). Cell counting analysis showed that indeed a significant higher percentage of PCK−/Vim+ cells were found in MESCM (14.8% ± 2.6%, n = 1352) when compared with cells expanded in SHEM (0.6 ± 0.3%, n = 1531, *P < 0.05). Such a difference was also confirmed by qPCR, which showed significant higher expression of Vim transcript in MESCM than in SHEM (Fig. 3B, **P < 0.01, n = 3). Although expression of Oct4, Nanog, and Sox2 transcripts was not significantly different between MESCM and SHEM (Fig. 3B), that of Rex1, Nestin, and N-cad as well as such markers of angiogenesis progenitors as FLK-1, CD34, CD31, and PDGFRβ, was significantly higher in MESCM than that in SHEM (Fig. 3C, **P < 0.01, *P < 0.05). In addition, expression of CXCR4, which we have reported to be expressed by limbal NCs,12 was also significantly higher in MESCM than SHEM (Fig. 3C, *P < 0.05). The difference in the protein expression of Rex1, PDGFRβ, and CD34 between MESCM and SHEM was further confirmed by immunostaining (Fig. 3C). Double immunostaining showed the presence of small PDGFRβ+/PCK− niche cells in the MESCM outgrowth but not that in the SHEM (Fig. 3C, white arrowhead). These results collectively suggested that MESCM indeed preserved a significant number of limbal NCs expressing their characteristic markers of ESC and angiogenesis progenitors12,13 better than SHEM on dAM.
Figure 3. .
MESCM preserves more PCK−/Vim+ NCs than SHEM. Both dispase-isolated limbal epithelial sheets (Dis) and collagenase-isolated limbal clusters (Coll) were cultured on dAM in SHEM or MESCM for 8 days. Double immunostaining between PCK (green) and Vim (red) of the resultant cytospin preparation revealed a significantly more Vim+/PCK− cells in MESCM than in SHEM (A, n = 1352 vs. 1531, *P < 0.05). When compared with the control derived from dispase-isolated sheet on day 0, qPCR showed no significant difference of ES marker and angiogenesis marker expressions between Dis/SHEM and Coll/SHEM (B). In contrast, Coll/MESCM showed significantly higher transcript expressions of Rex1, Nestin, N-cad, but not Oct4, Nanog, and Sox2 when compared with outgrowth in Coll/SHEM (B, n = 3, **P < 0.01). The transcript expression of angiogenesis progenitor markers, such as FLK-1, CD34, CD31, PDGFR-β, CXCR4, and NG2, was significantly higher in Coll/MESCM than in Coll/SHEM and Dis/SHEM (B, n = 3, *P < 0.05, **P < 0.01). Dash line marks 1 for the control of Dis/isolated sheet on day 0. Immunostaining showed more expression of Rex1, Nanog, PDGFR-β, and CD34 in Coll/MESCM than in Coll/SHEM (C) Small PDGFR-β+/PCK− cells were found in MESCM outgrowth but not SHEM outgrowth (C, white arrow head). Nuclei were counterstained by Hoechst 33342 (blue). Scale bar = 50 μm.
LEPC are Better Maintained by MESCM
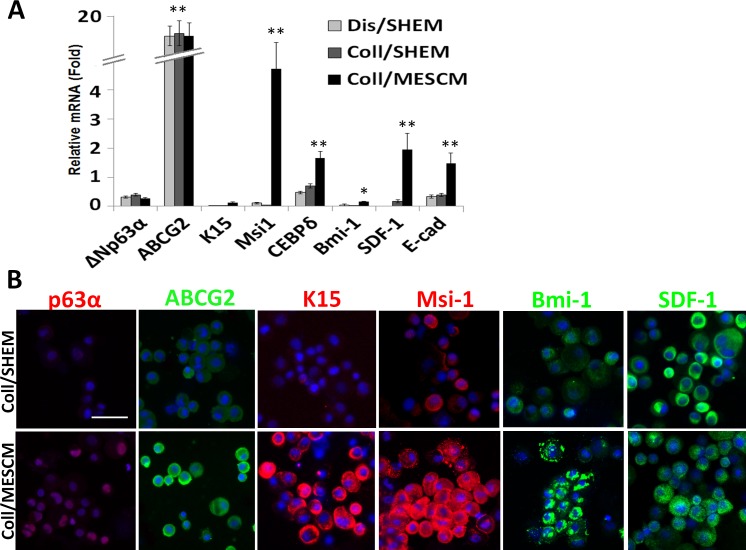
To determine whether LEPC were also better maintained because of the preservation of NCs in MESCM, we further examined the expression of putative limbal progenitor markers. qPCR showed that the expression of ΔNp63α,17 K15,18 Msi1,19 CEBPδ,20 Bmi-1,21 SDF-1,12 and E-cad transcripts were expressed by the outgrowth cultured in MESCM than that in SHEM (Fig. 4A, *P < 0.05, **P < 0.01, n = 3). There was no significant difference in expression of ABCG2 transcript22 between MESCM and SHEM when compared with the control of dispase-isolated limbal epithelial outgrowth, which contained predominant LEPC. Immunostaining confirmed stronger positive co-expression of ABCG2, K15, Msi1, and Bmi-1 by PCK epithelial cells in MESCM than that in SHEM (Fig. 4B). Immunostaining of nuclear expression of p63α and SDF-1 was not significantly different. These results supported the notion that the phenotype of LEPC was also better maintained by MESCM than SHEM.
Figure 4. .
MESCM preserves more LEPC expressing limbal SC markers. Dispase-isolated sheets or collagenase-isolated limbal clusters were cultured on dAM in MESCM or in SHEM for 8 days. Compared with the outgrowth derived from dispase-isolated limbal epithelial sheets in SHEM as the control, qPCR analysis showed a significantly higher expression of limbal epithelial SC markers such as ΔNp63a, K15, Msi1, CEBPδ, Bmi-1, SDF-1, and E-cad in outgrowth cultured in MESCM than that in SHEM (A, *P < 0.05, **P < 0.01, n = 3). Immunostaining also showed stronger positive expression of ABCG2, K15, Msi-1, and Bmi-1 in Coll/MESCM than Coll/SHEM (B). Scale bar = 50 μm.
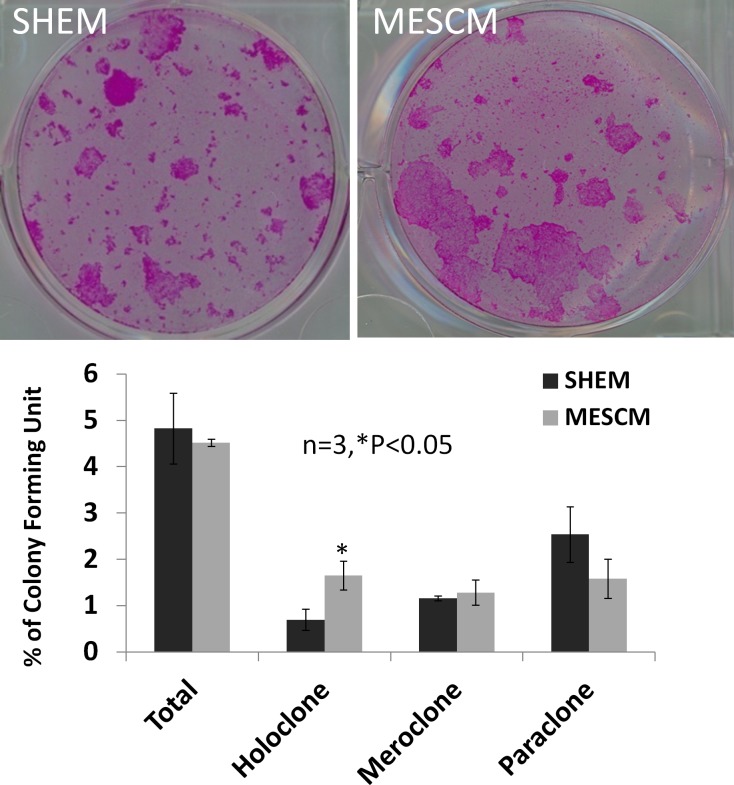
Higher Percentage Colony Forming Efficiency Observed in MESCM than in SHEM
To further substantiate the notion that MESCM maintained limbal NCs and LEPC better than SHEM, we examined the resultant clonal growth on mitotically-arrested 3T3 fibroblast feeder layers. Because MESCM maintained more PCK−/Vim+ cells, we normalized the number of resultant clones by the same number of PCK+/Vim cells. The total percentage of colony forming efficiency was not different between SHEM and MESCM (4.8 ± 0.7% vs. 4.1 ± 0.4%, n = 3). However, outgrowth cultured in MESCM showed a significantly higher number of purported holoclones than that cultured in SHEM (0.7 ± 0.2% vs. 1.2 ± 0.3%, n = 3, P < 0.05). There was no significant difference regarding the numbers of meroclone (1.6 ± 0.2% vs. 1.3 ± 0.3%, P > 0.05) and paraclone (2.4 ± 0.5% vs. 1.6 ± 0.4%, P > 0.05) between MESCM and SHEM.
Discussion
The present study lends stronger support to the idea that ex vivo expansion of LEPC can be further optimized by maintaining limbal NCs that have been included in collagenase-isolated limbal clusters that have been recently reported.11 The concept that the function of LEPC can be better promoted by maintaining close contact with limbal NCs was first demonstrated in a clonal assay in a serum-free medium and in an outgrowth on dAM in SHEM.11 Subsequently, this concept was proven by re-establishing the close contact between LEPC and limbal NCs, of which the in vivo close contact between these two cells had been intentionally disrupted, in a re-union assay on 3D Matrigel in MESCM.11,12 In latter studies, we noted that the phenotype of limbal NCs can be threatened by culturing on a substrate other than Matrigel12,13 and/or in a medium containing serum such as SHEM or DMEM plus 10% FBS.12 Hence, the present study was carried out to further prove the concept by switching SHEM to MESCM during ex vivo expansion of LEPC on dAM using collagenase-isolated limbal clusters, which have included both LEPC and limbal NCs and their close contact from the beginning.
Our results demonstrated that the simple maneuver of switching from SHEM to MESCM has effectively circumvented the demise of losing the phenotypes of limbal NCs and LEPC during ex vivo expansion on dAM (Fig. 1). Although the epithelial outgrowth cultured on dAM in MESCM was smaller than that cultured in SHEM during the second week of expansion (Fig. 2), the resultant epithelial outgrowth contained a significantly higher number of PCK−/Vim+ cells (Fig. 3). These PCK−/Vim+ cells were found to be limbal NCs because of upregulation of a number of ESC markers such as Rex1, Nestin, and N-cad as well as such markers of angiogenesis progenitors as FLK-1, CD34, CD31, and PDGFRβ (Fig. 3). In addition, expression of CXCR4, which we have reported to be expressed by limbal NCs,23 was also significantly higher in MESCM than SHEM (Fig. 3). Consequently, the phenotype of LEPC was also better preserved as evidenced by significant upregulation of markers of LEPC and/or SCs such as ΔNp63α,17 K15,18 Msi1,19 CEBPδ,20 Bmi-1,21 and SDF-1.23 Moreover, the resultant epithelial outgrowth exhibited a significantly higher number of purported holoclones in MESCM than SHEM (Fig. 5).
Figure 5. .
MESCM maintains LEPC generating more purported holoclones. Outgrowth expanded on dAM in SHEM or MESCM for 7 days was harvested by Trypsin/EDTA. A total of 1000 single cells were seeded on 3T3 feeder layer for 12 days. The colony forming efficiency was normalized to the same number of PCK+ cells. MESCM resulted in a significantly higher number of purported holoclones than SHEM (n = 3, *P < 0.05). There was no significant difference in the total colony forming efficiency along with difference in number of meroclones and paraclones between MESCM and SHEM (n = 3, P > 0.05).
Taken together, we believe that the ex vivo protocol of expanding LEPC on dAM can further be optimized by switching the medium from SHEM to MESCM. Continuous pursuits in this direction by focusing on better maintenance of limbal NCs and their close interaction with LEPC should generate new strategies to further improve the efficiency of expanding a surgical graft that contains LEPC to correct corneal blindness caused by a number of ocular surface diseases that manifest limbal SC deficiency.
Acknowledgments
The authors thank Angela Tseng for excellent technical assistance.
Supported by a National Eye Institute/National Institutes of Health Grant RO1 EY06819 (SCGT).
Disclosure: S.-Y. Chen, TissueTech, Inc. (E); M. Mahabole, TissueTech, Inc. (E); S.C.G. Tseng, TissueTech, Inc. (E), National Eye Institute, National Institutes of Health (F)
References
- 1.Greco V, Guo S. Compartmentalized organization: a common and required feature of stem cell niches? Development. 2010;137:1586–1594. doi: 10.1242/dev.041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li W, Hayashida Y, Chen YT, Tseng SC. Niche regulation of corneal epithelial stem cells at the limbus. Cell Res. 2007;17:26–36. doi: 10.1038/sj.cr.7310137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lavker RM, Tseng SC, Sun TT. Corneal epithelial stem cells at the limbus: looking at some old problems from a new angle. Exp Eye Res. 2004;78:433–446. doi: 10.1016/j.exer.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Endo K, Nakamura T, Kawasaki S, Kinoshita S. Human amniotic membrane, like corneal epithelial basement membrane, manifests the alpha5 chain of type IV collagen. Invest Ophthalmol Vis Sci. 2004;45:1771–1774. doi: 10.1167/iovs.03-0952. [DOI] [PubMed] [Google Scholar]
- 5.Fukuda K, Chikama T, Nakamura M, Nishida T. Differential distribution of subchains of the basement membrane components type IV collagen and laminin among the amniotic membrane, cornea, and conjunctiva. Cornea. 1999;18:73–79. [PubMed] [Google Scholar]
- 6.Shimazaki J, Yang H-Y, Tsubota K. Amniotic membrane transplantation for ocular surface reconstruction in patients with chemical and thermal burns. Ophthalmology. 1997;104:2068–2076. doi: 10.1016/s0161-6420(97)30057-8. [DOI] [PubMed] [Google Scholar]
- 7.Koizumi N, Inatomi T, Quantock AJ, Fullwood NJ, Dota A, Kinoshita S. Amniotic membrane as a substrate for cultivating limbal corneal epithelial cells for autologous transplantation in rabbits. Cornea. 2000;19:65–71. doi: 10.1097/00003226-200001000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Touhami A, Grueterich M, Tseng SC. The role of NGF signaling in human limbal epithelium expanded by amniotic membrane culture. Invest Ophthalmol Vis Sci. 2002;43:987–994. [PubMed] [Google Scholar]
- 9.Grueterich M, Espana EM, Tseng SC. Ex vivo expansion of limbal epithelial stem cells: amniotic membrane serving as a stem cell niche. Surv Ophthalmol. 2003;48:631–646. doi: 10.1016/j.survophthal.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Tseng SC, Chen SY, Shen YC, Chen WL, Hu FR. Critical appraisal of ex vivo expansion of human limbal epithelial stem cells. Curr Mol Med. 2010;10:841–850. doi: 10.2174/156652410793937796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen SY, Hayashida Y, Chen MY, Xie HT, Tseng SCA. New isolation method of human limbal progenitor cells by maintaining close association with their niche cells. Tissue Eng Part C Methods. 2011;17:537–548. doi: 10.1089/ten.tec.2010.0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie HT, Chen SY, Li GG, Tseng SC. Isolation and expansion of human limbal stromal niche cells. Invest Ophthalmol Vis Sci. 2012;53:279–286. doi: 10.1167/iovs.11-8441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li GG, Chen SY, Xie HT, Zhu YT, Tseng SC. Angiogenesis potential of human limbal stromal niche cells. Invest Ophthalmol Vis Sci. 2012;53:3357–3367. doi: 10.1167/iovs.11-9414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Espana EM, Romano AC, Kawakita T, Di Pascuale M, Smiddy R, Tseng SC. Novel enzymatic isolation of an entire viable human limbal epithelial sheet. Invest Ophthalmol Vis Sci. 2003;44:4275–4281. doi: 10.1167/iovs.03-0089. [DOI] [PubMed] [Google Scholar]
- 15.Meller D, Tseng SCG. Conjunctival epithelial cell differentiation on amniotic membrane. Invest Ophthalmol Vis Sci. 1999;40:878–886. [PubMed] [Google Scholar]
- 16.Barrandon Y, Green H. Three clonal types of keratinocyte with different capacities for multiplication. Proc Natl Acad Sci U S A. 1987;84:2302–2306. doi: 10.1073/pnas.84.8.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Iorio E, Barbaro V, Ruzza A, Ponzin D, Pellegrini G, De LM. Isoforms of DeltaNp63 and the migration of ocular limbal cells in human corneal regeneration. Proc Natl Acad Sci U S A. 2005;102:9523–9528. doi: 10.1073/pnas.0503437102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshida S, Shimmura S, Kawakita T, et al. Cytokeratin 15 can be used to identify the limbal phenotype in normal and diseased ocular surfaces. Invest Ophthalmol Vis Sci. 2006;47:4780–4786. doi: 10.1167/iovs.06-0574. [DOI] [PubMed] [Google Scholar]
- 19.Chen YT, Li W, Hayashida Y, et al. Human amniotic epithelial cells as novel feeder layers for promoting ex vivo expansion of limbal epithelial progenitor cells. Stem Cells. 2007;25:1995–2005. doi: 10.1634/stemcells.2006-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barbaro V, Testa A, Di IE, Mavilio F, Pellegrini G, De LM. C/EBPdelta regulates cell cycle and self-renewal of human limbal stem cells. J Cell Biol. 2007;177:1037–1049. doi: 10.1083/jcb.200703003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Umemoto T, Yamato M, Nishida K, Yang J, Tano Y, Okano T. Limbal epithelial side-population cells have stem cell-like properties, including quiescent state. Stem Cells. 2006;24:86–94. doi: 10.1634/stemcells.2005-0064. [DOI] [PubMed] [Google Scholar]
- 22.Zhou S, Morris JJ, Barnes Y, Lan L, Schuetz JD, Sorrentino BP. Bcrp1 gene expression is required for normal numbers of side population stem cells in mice, and confers relative protection to mitoxantrone in hematopoietic cells in vivo. Proc Natl Acad Sci U S A. 2002;99:12339–12344. doi: 10.1073/pnas.192276999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie HT, Chen SY, Li GG, Tseng SC. Limbal epithelial stem/progenitor cells attract stromal niche cells by SDF-1/CXCR4 signaling to prevent differentiation. Stem Cells. 2011;29:1874–1885. doi: 10.1002/stem.743. [DOI] [PubMed] [Google Scholar]