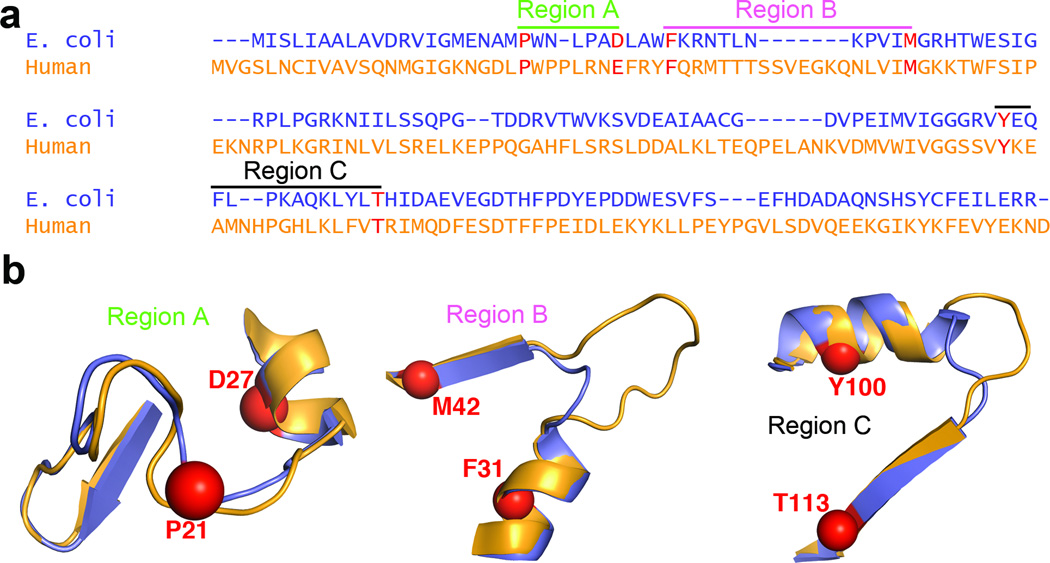
Figure 3. Primary sequence features related to flexibility and conformational change in E. coli and human DHFR.
(a) Sequence alignment of ecDHFR and hDHFR showing three regions of the sequence related to dynamic mechanism. The anchor residues for sequence alignment are shown in red. (b) Structure of regions highlighted in a, with anchor residues shown as spheres. ecDHFR is shown in purple, and hDHFR in orange. Regions A, B and C correspond to the “Met20” loop, hinge 1 and hinge 2, respectively. The following anchor residues were chosen for sequence alignments (E. coli numbering): P21 and D27 for Region A, F31 and M42 for Region B, and Y100 and T113 for Region C.