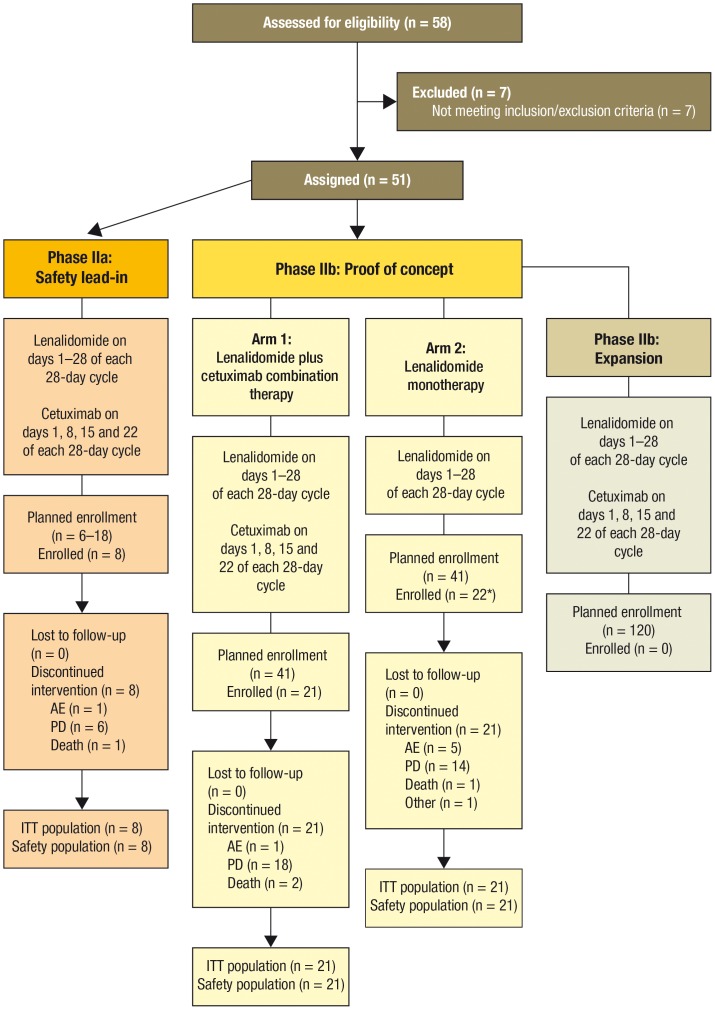
Figure 1. Study design and enrollment in patient groups.
Study was terminated before the expansion part of phase IIb. *One patient was randomized to the lenalidomide monotherapy group but discontinued before taking any study drug and was therefore excluded from the analyses. AE, adverse event; ITT, intention to treat; PD, progressive disease.