Abstract
The potential for carcinogenic risks is increased by radiation-induced bystander responses; these responses are the biological effects in unirradiated cells that receive signals from the neighboring irradiated cells. Bystander responses have attracted attention in modern radiobiology because they are characterized by non-linear responses to low-dose radiation. We used a synchrotron X-ray microbeam irradiation system developed at the Photon Factory, High Energy Accelerator Research Organization, KEK, and showed that nitric oxide (NO)-mediated bystander cell death increased biphasically in a dose-dependent manner. Here, we irradiated five cell nuclei using 10 × 10 µm2 5.35 keV X-ray beams and then measured the mutation frequency at the hypoxanthine-guanosine phosphoribosyl transferase (HPRT) locus in bystander cells. The mutation frequency with the null radiation dose was 2.6 × 10–5 (background level), and the frequency decreased to 5.3 × 10–6 with a dose of approximately 1 Gy (absorbed dose in the nucleus of irradiated cells). At high doses, the mutation frequency returned to the background level. A similar biphasic dose-response effect was observed for bystander cell death. Furthermore, we found that incubation with 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (carboxy-PTIO), a specific scavenger of NO, suppressed not only the biphasic increase in bystander cell death but also the biphasic reduction in mutation frequency of bystander cells. These results indicate that the increase in bystander cell death involves mechanisms that suppress mutagenesis. This study has thus shown that radiation-induced bystander responses could affect processes that protect the cell against naturally occurring alterations such as mutations.
Keywords: radiation-induced bystander responses, mutation, cell death, nitric oxide, microbeam irradiation
INTRODUCTION
Biological responses to radiation are induced in irradiated cells mainly as a result of DNA damage. However, many studies indicate that biological responses to radiation are not always limited to the irradiated cells but can be induced in neighboring unirradiated ‘bystander’ cells. This phenomenon, often called radiation-induced bystander response, was first described by Nagasawa and Little in 1992 [1]. So far the range of biological effects demonstrated to be induced in bystander cells via signals from irradiated cells includes sister chromatid exchange [1, 2], cell death [3–8], chromosomal instability [9], and mutations [10, 11]. These findings have had a large impact on radiobiology because they may have important implications for the estimation of risk to human health associated with exposure to low-dose radiation. The risks associated with low-dose ionizing radiation are estimated by extrapolating data obtained after exposure to intermediate doses using a linear non-threshold (LNT) model. The discovery of radiation-induced bystander responses and other non-targeted effects has triggered a dispute over the validity of the LNT model because a non-linear response at a low dose is a characteristic of these phenomena. The controversy over this issue has not been resolved, in part, because the number and location of the radiation-track traversals cannot be monitored or controlled for each cell when a broad radiation field is used, which is often true in low-dose radiation experiments. The microbeam cell irradiation system, which enables observation of cellular responses of individual irradiated and non-irradiated cells equally, is a powerful tool to elucidate the mechanisms underlying the biological responses to low-dose radiation, including bystander responses.
We used a synchrotron X-ray microbeam irradiation system developed at the Photon Factory, High Energy Accelerator Research Organization, KEK [12–15], and found that cell death is more prevalent in cells irradiated with X-ray microbeams when only nuclei, rather than the whole cells, are irradiated [16, 17]. Furthermore, we recently showed that the biphasic increase in bystander cell death was dose-dependent when nuclei of targeted cells were exposed to X-ray microbeams [7, 18]. Our findings indicated that cell death, both in irradiated and bystander cells, was modified by the site of energy deposition within the cells.
As a next step, we measured the mutation frequency in bystander cells neighboring those with irradiated nuclei. Because mutations are a prerequisite of carcinogenesis, our results may provide fundamental information for evaluating the carcinogenic risk posed by exposure to low doses of ionizing radiation.
MATERIALS AND METHODS
Cell culture and sample preparation
V79 Chinese hamster lung cells were cultured in minimum essential medium-alpha (MEMα; Nacalai Tesque Inc., Nakagyo-ku, Kyoto, Japan) containing 10% fetal bovine serum (FBS; Nichirei Biosciences Inc., Chuo-ku, Tokyo, Japan), 100 U/ml penicillin (Invitrogen Corp, Carlsbad, California, USA), 100 µg/ml streptomycin (Invitrogen), and 15 mM 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES; Nacalai Tesque) and then incubated in a humidified incubator maintained at 37°C in an atmosphere containing 5% CO2. To irradiate cells with microbeams, 1.0 × 105 V79 cells were seeded on custom-designed polypropylene-based dishes (34 mm in diameter), the bottoms of which were composed of a 3-µm polypropylene film (Toray Industries Inc., Chuo-ku, Tokyo, Japan), and incubated overnight. Before X-ray irradiation, the cell nuclei were stained with a 2 µM solution of Hoechst 33258 (Dojindo Molecular Technologies Inc., Kamimashiki-gun, Kumamoto, Japan) for 1 h. At the time of irradiation, the Hoechst solution was replaced with 5 ml of fresh medium.
Microbeam irradiation
Monochromatic X-ray microbeam irradiation was performed using the synchrotron X-ray microbeam irradiation system installed at the BL-27B station in the Photon Factory [12–15]. The procedures for microbeam irradiation and dosimetry have been previously described [7, 17]. To ensure that the irradiation for initial stimulation was similar to that used in previous studies [7, 18], nuclei of five isolated single cells located at the center of each dish were selected as targets. The positions of these nuclei, defined as the center of mass of Hoechst33258-stained nuclei images, were stored in the controlling computer of the system. We irradiated the five targeted cell nuclei with 10 × 10 µm2 5.35 keV X-ray beams, and the exposure rates were 8.5 × 10−3 ± 3.4 × 10−5 C/kg/s (1.0 × 104 ± 4.2 × 101 photons/s in 10 × 10 µm2 beam). In our study, the ‘nuclear-averaged dose’, at which the absorbed energy is divided by the mass of nucleus as described in Maeda et al. [17], was used as a measure of radiation doses and the dose rate was 1.8 × 10−1 ± 7.3 × 10−4 Gy/s.
Determination of bystander cell survival
The fraction of bystander cells that survived was measured using a colony-formation assay. After irradiation, the culture medium was removed, and the cells were washed twice with phosphate buffered saline (PBS). Immediately thereafter, 2 ml of fresh medium was added to the dishes, and the cells were cultured for 3 h. Next the cells were harvested using a trypsin-ethylene diamine tetraacetic acid (EDTA) solution (0.05% trypsin, 0.53 mM EDTA•4Na; Invitrogen); the harvested cells were diluted and plated in a 100-mm cell culture dish at approximately 150 viable cells per dish. After incubation for 6 days, the cells were fixed with HC Tissue Fixative MB (Amresco Inc., Solon, Ohio, USA) for 25 min at room temperature and rinsed twice with PBS. Following that the cells were stained with 1% methylene blue (Wako Pure Chemical Industries Ltd, Chuo-ku, Osaka, Japan) solution. Colonies containing more than 50 cells were counted as survivors.
HPRT mutation assay
The HPRT mutation assay is a method commonly used to study the genetic changes and genomic instability [19, 20]. After irradiation, the culture medium was removed, and the cells were washed twice with PBS. Immediately thereafter, 2 ml of fresh medium was added to the dishes, and the cells were cultured for 3 h. Cells were harvested by trypsinization and transferred to a T-75 cell culture flask containing fresh medium. Cells were maintained for 8 days and were subcultivated every 2 days to allow for phenotypic expression. Then, 1 × 106 cells were harvested and seeded onto 100-mm cell culture dishes with fresh medium containing 10 µg/ml 6-thioguanine (Wako) and incubated for 6 days. Cells in dishes were fixed and stained using the same method described above for the colony-formation assay, and the colonies (i.e. HPRT mutants) were scored. The mutation frequency was expressed as the number of resistant colonies divided by the total number of viable cells at the time of selection.
Cell culture with NO scavenger after irradiation
2-(4-Carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide, sodium salt (carboxy-PTIO; Dojindo Molecular Technologies Inc.) is a specific scavenger of NO [21, 22]. Directly after irradiation, the cells were incubated with medium containing carboxy-PTIO instead of normal fresh medium. During the clonogenic assays for the measurement of surviving fractions and of mutation frequencies, cells were also incubated with medium containing carboxy-PTIO instead of normal fresh medium. The concentration of carboxy-PTIO in the medium was set to 20 µM, because the concentration of NO in the medium was not expected to exceed 20 µM after irradiation [7], as indicated by studies in which the concentration of NO2−, an oxidization product of NO, in the medium after irradiation was measured with Griess reagent [23].
Statistical analysis
Statistical analysis was performed on the data obtained from at least three independent experiments. All the results are expressed as means ± standard error (SE). Significant levels were assessed using Student's t test. Analysis of the correlations between bystander cell death and mutation frequency in the bystander cells was assessed using analysis of variance (ANOVA). A probability (P) value < 0.05 was considered to indicate statistical significance.
RESULTS
Determination of the incubation period for the assays of bystander responses
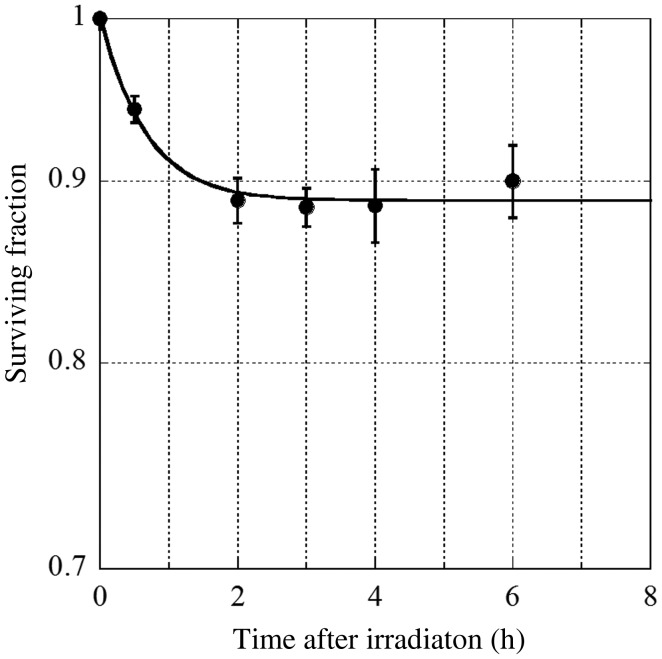
We first determined the incubation period for trypsinization to harvest the cells for the colony formation assay. Cell nuclei (n = 5) located in the center of a dish were irradiated with 1 Gy of 10 × 10 µm2 square 5.35 keV X-ray beams and incubated for 0–6 h. Then, the surviving fractions of the cell populations on the dishes were determined using the colony-formation assay. We set the incubation period as 3 h, because the decrease in surviving fractions reached a plateau 3 h after irradiation (Fig. 1).
Fig. 1.
The surviving fraction of bystander V79 cells is plotted as a function of the time after irradiation. Data were taken from more than three independent experiments. The error bars represent standard errors (SEs).
Bystander cell death induced by microbeam-irradiated V79 cells
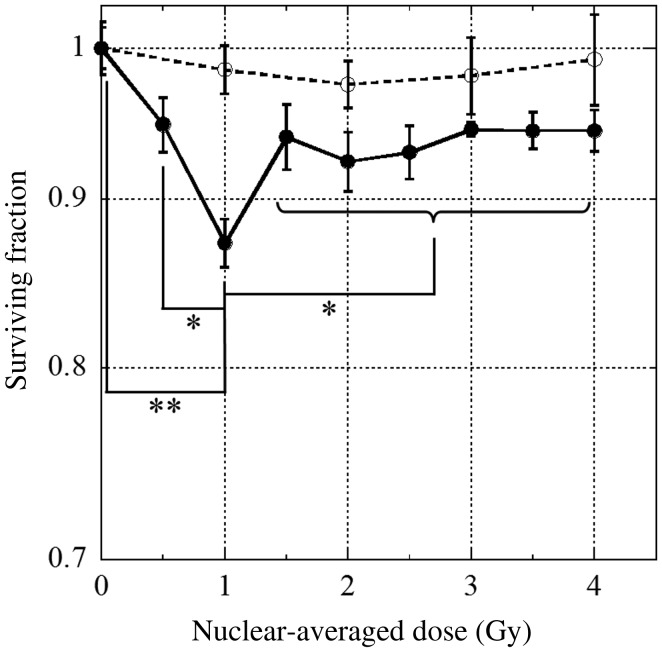
We measured the dose–survival relationship in bystander V79 cells (Fig. 2). We observed a dose-dependent biphasic increase in the death of bystander cells. The surviving fraction decreased to 0.87 ± 0.015 when nuclei were irradiated with a nuclear-averaged dose of 1 Gy; however, at higher doses the surviving fraction was stable at approximately 0.94. Because the fraction of irradiated cells in the dish was < 0.5 × 10−4, it is likely that the decrease in the surviving fraction was the result of bystander responses. Our previous studies showed the same type of biphasic dose–response effect as that observed in this study during the death of bystander cells [7, 8, 18].
Fig. 2.
The surviving fractions of bystander V79 cells are plotted as a function of the nuclear-averaged dose in the irradiated cells. Cells were incubated with (open circles) or without (closed circles) the nitric oxide (NO)-specific scavenger, 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (carboxy-PTIO), commencing immediately after irradiation. Data were taken from at least three independent experiments. The error bars represent standard errors (SEs). An asterisk indicates P < 0.05, and double asterisks indicate P < 0.01.
In the previous study, 2.0 × 103 individual V79 cells were seeded on dishes, and five cells at the center of the dish were irradiated [7, 18]. On the other hand, in the present study, 1.0 × 105 cells were seeded on dishes. Although, the shape of the dose–survival curves and the magnitude of bystander cell death were almost equal in both studies [7, 18], the fraction of irradiated cells among the cell population was 0.25% and 0.005%, respectively. These results indicate that initial small stimulation (i.e. irradiation to only five cell nuclei in the cell population) is sufficient to saturate the bystander cell death.
HPRT mutation in the bystander cell population
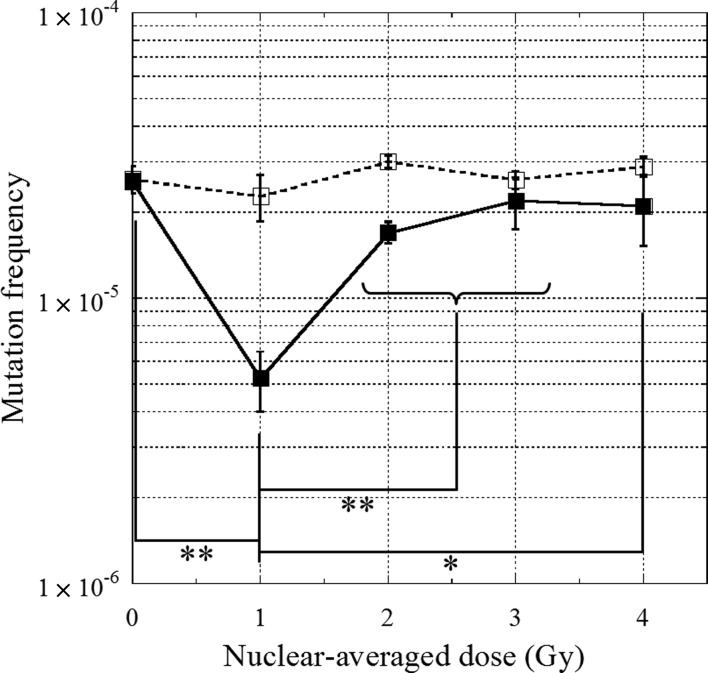
The dose–response relationship between radiation dose and mutation frequency in the bystander cell population was determined using the HPRT mutation assay (Fig. 3). The background mutation frequency in the control, non-irradiated cells, was 2.6 × 10−5 ± 1.3 × 10−6. The mutation frequency in bystander cells decreased significantly (P < 0.01) to 5.3 × 10−6 ± 1.3 × 10−6 when five target nuclei were irradiated with a nuclear-averaged dose of 1 Gy; however, at higher doses the mutation frequency returned to background levels. The biphasic decrease in mutation frequency was similar to the biphasic decrease in bystander cell death.
Fig. 3.
HPRT mutation frequencies in bystander cells are plotted as a function of the nuclear-averaged dose in the irradiated cells.Cells were incubated with (open squares) or without (closed squares) the nitric oxide (NO)-specific scavenger, 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (carboxy-PTIO), commencing immediately after irradiation. Data were taken from at least three independent experiments. The error bars represent standard errors (SEs). An asterisk indicates P < 0.05, and double asterisks indicate P < 0.01.
The effects of NO on bystander cell death and mutagenesis in the population of bystander cells
Recently, we showed that NO is a principal mediator of bystander cell death [7]. Therefore, we investigated the role of NO in bystander cell death and mutations. Treatment with 20 µM carboxy-PTIO, a specific scavenger of NO [21, 22], was not cytotoxic to V79 cells (Table 1). The effects of carboxy-PTIO in post-irradiation incubation are shown in Figs 2 and 3. The dose-dependent biphasic increase in the death of bystander cells was not observed when the cells were incubated with carboxy-PTIO (Fig. 2). Furthermore, the dose-dependent biphasic decrease in mutation frequency was not observed when the cells were incubated with carboxy-PTIO (Fig. 3). These results clearly show that NO plays an important role, not only in the induction of death of bystander cells, but also in the suppression of spontaneous mutagenesis in bystander cells.
Table 1.
Cytotoxicity of incubating V79 cells with 20 µM 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (carboxy-PTIO)
| carboxy-PTIO (20 µM) | – | + |
|---|---|---|
| Colony-forming efficiency | 0.92 (±0.070) | 0.91 (±0.050) |
| Mutation frequency | 2.6 × 10−5 (±7.8 × 10−6) | 2.6 × 10−5 (±6.0 × 10−6) |
DISCUSSION
Our findings showed a biphasic dose–response relationship between the mutation frequency in bystander cells and irradiation dose when the nuclei of targeted cells were exposed to X-ray microbeams. Previous studies on bystander responses, especially those investigating mutations in bystander cells, mostly focused on the effects caused by irradiation with low fluences of α-particles. For example, Nagasawa and Little found an unexpectedly high frequency of HPRT mutations in CHO cells exposed to very low fluences of α-particles; they concluded that the mutations occurred in unirradiated cells via bystander responses [10]. Similarly, Zhou et al. reported that when 20% of human-hamster hybrid (AL) cells were irradiated with 20 α-particles using charged particle microbeams, the frequency of mutations in the human chromosome 11 in the other 80% of the cells (i.e. bystander cells) quadrupled relative to background levels [11]. Our findings, unlike those from previous studies, showed that the HPRT mutation frequency in bystander V79 cells decreased at radiation doses around 1 Gy (Fig. 3). The mutation frequency significantly decreased (P < 0.01) from 2.6 × 10−5 (background level) to 5.3 × 10−6 at a nuclear-averaged dose of approximately 1 Gy; however, at higher doses the mutation frequency returned to the background level. Interestingly, this dose–response effect is quite similar to that observed in the death of bystander cells (Fig. 2). ANOVA indicated a significant correlation (P < 0.05) between bystander cell death and HPRT mutation frequency. The similarity of these behaviors indicates the possibility that bystander cell death and mutations in bystander cells are not independent phenomena.
Some groups reported a similar dose–response in neoplastic transformation after exposure to low-dose radiation. Although the phenomena described in those studies are not bystander responses, the discussions in those reports might be useful to help consider the mechanisms underlying our results. Azzam et al. reported that low-dose γ-ray irradiation reduces the risk of neoplastic transformation from the spontaneous level to one-third or one-fourth of that level [24]. They proposed that exposure to low doses of radiation resulted in an increased capacity for the error-free repair of DNA double-strand breaks [24]. In addition, Redpath and his colleagues reported that low-dose γ-ray or X-ray irradiation protected a human hybrid cell line against spontaneous neoplastic transformation. Interestingly, the dose–response in their work was biphasic, similar to that observed in our HPRT mutation study. They suggested that a reduction in the oxidative stress, possibly as a consequence of the upregulation of antioxidants by low doses of irradiation, might be a mechanism mediating those phenomena [25, 26]. Because in our study the viability of bystander cells decreased in the same dose range as that of the reduction of HPRT mutation frequency, the activation of antioxidant functions, rather than the upregulation of DNA repair capability, may be related to the suppression of mutagenesis. The oxidative damage of nucleotides within DNA or precursor pools that is caused by reactive oxygen species (ROS) is thought to play an important role in spontaneous mutations. Intracellular levels of ROS are persistently high in genetically unstable cells [27–29], and clones of those unstable cells secrete factors such as cytokines and persistent free radicals that contribute to the perpetuation of the unstable phenotype [30]. Bystander responses might suppress the secretion of those factors and/or elevate the antioxidant functions in the bystander cells population.
To date, two major classes of intercellular signaling events, direct cell-to-cell contact via gap junctions [11, 31–33] and indirect communication via secreted factors [34, 35], are known to be involved in the induction of bystander responses. Hou et al. reported that most mutations induced in bystander cells were point mutations and suggested that these mutations may have been caused by ROS [36]. However, Zhou et al. reported that oxidative stress or hydroxyl radicals, which have a very short half-life, were not chief mediators of mutations in bystander cells [11]; they showed that the inhibition of gap-junction communication suppressed the mutagenesis in bystander cells [11, 37]. V79 cells, the cells used in this study, exhibit some degree of gap-junction intercellular communication [38, 39]. However, gap junction-mediated bystander responses may have been rare in our study because we seeded the cells on the irradiation dish at low densities, and most cells did not contact other cells during the short incubation periods after irradiation. Therefore, it is likely that the observed bystander responses were due to indirect communication mediated by secreted factors. Certain bystander signaling factors secreted from irradiated cells, such as NO [7, 8, 40–45] and transforming growth factor-β1 (TGF-β1) [46–48], apparently act as mediators between irradiated and bystander cells. Recently, we showed that NO is a principal mediator of bystander cell death; the addition of carboxy-PTIO, a specific scavenger of NO, to the culture medium suppresses the biphasic bystander cell death [7]. The dose-dependent biphasic increase in the death of bystander cells was suppressed by scavenging of the NO in a similar manner (Fig. 2). Interestingly, scavenging of the NO also suppresses the dose-dependent biphasic decrease of the mutation frequency in bystander cells (Fig. 3). These results clearly indicate an increase in NO-mediated bystander cell death participated in mechanisms that suppressed mutagenesis in bystander cells. In many cases [4–8, 49], approximately 5–20% of the bystander cells were killed by radiation-induced bystander responses. However, it is not clear why bystander responses induce cell death at those frequencies. Recently, Egashira et al. reported that exposure to NO causes mitochondrial degeneration and subsequent cell killing in cells that have low antioxidative functions [50]. Because NO is a major mediator of bystander cell death, the cells that are genetically unstable because of defects in their antioxidative activity might be selectively killed by bystander responses. Thus, the secretion of factors that contributed to the perpetuation of the unstable phenotype may have been suppressed, and the antioxidant activity in surviving cells may have been increased; therefore, mutagenesis may have been suppressed in the bystander cells (Fig. 3). Our group has reported that the biphasic NO-mediated bystander cell death was induced by X-ray microbeams also in normal human fibroblast WI-38 cells [8]. A reduction in the mutation frequency may occur in normal human bystander cells if NO-mediated bystander cell death selectively kills genetically unstable cells.
Radiation-induced bystander responses are generally thought to increase the risk of low-dose radiation to human health because many cytotoxic phenomena are observed in bystander cells. However, previous studies, as well as this study, have reported the presence of a radiation-protective bystander phenomena [48, 51, 52]. These phenomena are part of the cellular homeostatic response and it is difficult to categorize these effects as beneficial or harmful. To assess the effects of radiation-induced bystander responses on human health precisely, it is necessary not only to elucidate the mechanisms mediating individual endpoints, but also to understand the biological significances for the one living system. Our results indicated that radiation-induced bystander responses can enhance selective cell killing of genetically unstable cells in the bystander cell population, and this selective cell death might act as a protective mechanism that competes with increases in non-lethal and potentially carcinogenic damage (e.g. mutations). These hypothetical protective effects may provide an adaptive advantage to the organism.
FUNDING
This work was supported in part by a Grant-in-Aid for Young Scientists (A) (21681006) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan, and a Grant-in-Aid for Young Scientists (B) (23710076) from Japan Society for the Promotion of Science (JSPS).
ACKNOWLEDGEMENTS
The authors would like to express their sincere gratitude to Drs Mika Maeda, Hiroshi Maezawa and Kensuke Otsuka for their valuable discussions concerning this study. We thank Ms Masako Mizuno for her technical assistance. The study was approved by the Photon Factory Program Advisory Committee (proposal numbers 2008G624 and 2010G040).
REFERENCES
- 1.Nagasawa H, Little JB. Induction of sister chromatid exchanges by extremely low doses of alpha-particles. Cancer Res. 1992;52:6394–6. [PubMed] [Google Scholar]
- 2.Deshpande A, Goodwin EH, Bailey SM, et al. Alpha-particle-induced sister chromatid exchange in normal human lung fibroblasts: evidence for an extranuclear target. Radiat Res. 1996;145:260–7. [PubMed] [Google Scholar]
- 3.Sawant SG, Randers-Pehrson G, Geard CR, et al. The bystander effect in radiation oncogenesis: I. Transformation in C3H 10T1/2 cells in vitro can be initiated in the unirradiated neighbors of irradiated cells. Radiat Res. 2001;155:397–401. doi: 10.1667/0033-7587(2001)155[0397:tbeiro]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 4.Sawant SG, Randers-Pehrson G, Metting NF, et al. Adaptive response and the bystander effect induced by radiation in C3H 10T(1/2) cells in culture. Radiat Res. 2001;156:177–80. doi: 10.1667/0033-7587(2001)156[0177:aratbe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 5.Sawant SG, Zheng W, Hopkins KM, et al. The radiation-induced bystander effect for clonogenic survival. Radiat Res. 2002;157:361–4. doi: 10.1667/0033-7587(2002)157[0361:tribef]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 6.Schettino G, Folkard M, Michael BD, et al. Low-dose binary behavior of bystander cell killing after microbeam irradiation of a single cell with focused Ck X rays. Radiat Res. 2005;163:332–6. doi: 10.1667/rr3319. [DOI] [PubMed] [Google Scholar]
- 7.Maeda M, Tomita M, Usami N, et al. Bystander cell death is modified by sites of energy deposition within cells irradiated with a synchrotron X-ray microbeam. Radiat Res. 2010;174:37–45. doi: 10.1667/RR2086.1. [DOI] [PubMed] [Google Scholar]
- 8.Tomita M, Maeda M, Maezawa H, et al. Bystander cell killing in normal human fibroblasts is induced by synchrotron X-ray microbeams. Radiat Res. 2010;173:380–5. doi: 10.1667/RR1995.1. [DOI] [PubMed] [Google Scholar]
- 9.Lorimore SA, Kadhim MA, Pocock DA, et al. Chromosomal instability in the descendants of unirradiated surviving cells after alpha-particle irradiation. Proc Natl Acad Sci U S A. 1998;95:5730–3. doi: 10.1073/pnas.95.10.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagasawa H, Little JB. Unexpected sensitivity to the induction of mutations by very low doses of alpha-particle radiation: evidence for a bystander effect. Radiat Res. 1999;152:552–7. [PubMed] [Google Scholar]
- 11.Zhou H, Randers-Pehrson G, Waldren CA, et al. Induction of a bystander mutagenic effect of alpha particles in mammalian cells. Proc Natl Acad Sci U S A. 2000;97:2099–104. doi: 10.1073/pnas.030420797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobayashi K, Usami N, Hieda K, et al. Development of microbeam irradiation system for radiobiology. Nucl Instrum Meth A. 2001:467–468. 1329–32. [Google Scholar]
- 13.Kobayashi K, Usami N, Maezawa H, et al. Development of photon microbeam irradiation system for radiobiology. Int Congr Ser. 2003;1258:207–11. [Google Scholar]
- 14.Kobayashi K, Usami N, Maezawa H, et al. Synchrotron X-ray microbeam irradiation system for radiobiology. J Biomed Nanotechnol. 2006;2:116–9. [Google Scholar]
- 15.Kobayashi Y, Funayama T, Hamada N, et al. Microbeam irradiation facilities for radiobiology in Japan and China. J Radiat Res. 2009;50(Suppl A):A29–47. doi: 10.1269/jrr.09009s. [DOI] [PubMed] [Google Scholar]
- 16.Maeda M, Usami N, Kobayashi K. Cell survival study using KEK-PF synchrotron X-ray microbeam irradiation system. Radiat Res. 2006;166:679–80. [Google Scholar]
- 17.Maeda M, Usami N, Kobayashi K. Low-dose hypersensitivity in nucleus-irradiated V79 cells studied with synchrotron X-ray microbeam. J Radiat Res. 2008;49:171–80. doi: 10.1269/jrr.07093. [DOI] [PubMed] [Google Scholar]
- 18.Maeda M, Tomita M, Kobayashi K. Study of the relationship between bystander cell death and intracellular energy-deposited sites using a KEK-PF SR X-ray microbeam irradiation system. J Radiat Res. 2009;50(Suppl A):A119. [Google Scholar]
- 19.Nestmann ER, Brillinger RL, Gilman JP, et al. Recommended protocols based on a survey of current practice in genotoxicity testing laboratories: II. Mutation in Chinese hamster ovary, V79 Chinese hamster lung and L5178Y mouse lymphoma cells. Mutat Res. 1991;246:255–84. doi: 10.1016/0027-5107(91)90048-s. [DOI] [PubMed] [Google Scholar]
- 20.Little JB, Frenial JM, Coppey J. Studies of mutagenesis and neoplastic transformation by bivalent metal ions and ionizing radiation. Teratog Carcinog Mutagen. 1988;8:287–92. doi: 10.1002/tcm.1770080505. [DOI] [PubMed] [Google Scholar]
- 21.Akaike T, Yoshida M, Miyamoto Y, et al. Antagonistic action of imidazolineoxyl N-oxides against endothelium-derived relaxing factor/.NO through a radical reaction. Biochemistry. 1993;32:827–32. doi: 10.1021/bi00054a013. [DOI] [PubMed] [Google Scholar]
- 22.Pfeiffer S, Leopold E, Hemmens B, et al. Interference of carboxy-PTIO with nitric oxide- and peroxynitrite-mediated reactions. Free Radic Biol Med. 1997;22:787–94. doi: 10.1016/s0891-5849(96)00407-8. [DOI] [PubMed] [Google Scholar]
- 23.Green LC, Wagner DA, Glogowski J, et al. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem. 1982;126:131–8. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 24.Azzam EI, de Toledo SM, Raaphorst GP, et al. Low-dose ionizing radiation decreases the frequency of neoplastic transformation to a level below the spontaneous rate in C3H 10T1/2 cells. Radiat Res. 1996;146:369–73. [PubMed] [Google Scholar]
- 25.Redpath JL. Suppression of neoplastic transformation in vitro by low doses of low LET radiation. Dose Response. 2006;4:302–8. doi: 10.2203/dose-response.06-114.Redpath. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elmore E, Lao XY, Kapadia R, et al. Low doses of very low-dose-rate low-LET radiation suppress radiation-induced neoplastic transformation in vitro and induce an adaptive response. Radiat Res. 2008;169:311–8. doi: 10.1667/RR1199.1. [DOI] [PubMed] [Google Scholar]
- 27.Clutton SM, Townsend KM, Walker C, et al. Radiation-induced genomic instability and persisting oxidative stress in primary bone marrow cultures. Carcinogenesis. 1996;17:1633–9. doi: 10.1093/carcin/17.8.1633. [DOI] [PubMed] [Google Scholar]
- 28.Limoli CL, Hartmann A, Shephard L, et al. Apoptosis, reproductive failure, and oxidative stress in Chinese hamster ovary cells with compromised genomic integrity. Cancer Res. 1998;58:3712–8. [PubMed] [Google Scholar]
- 29.Limoli CL, Kaplan MI, Giedzinski E, et al. Attenuation of radiation-induced genomic instability by free radical scavengers and cellular proliferation. Free Radic Biol Med. 2001;31:10–9. doi: 10.1016/s0891-5849(01)00542-1. [DOI] [PubMed] [Google Scholar]
- 30.Morgan WF, Hartmann A, Limoli CL, et al. Bystander effects in radiation-induced genomic instability. Mutat Res. 2002;504:91–100. doi: 10.1016/s0027-5107(02)00083-0. [DOI] [PubMed] [Google Scholar]
- 31.Azzam EI, de Toledo SM, Gooding T, et al. Intercellular communication is involved in the bystander regulation of gene expression in human cells exposed to very low fluences of alpha particles. Radiat Res. 1998;150:497–504. [PubMed] [Google Scholar]
- 32.Bishayee A, Rao DV, Howell RW. Evidence for pronounced bystander effects caused by nonuniform distributions of radioactivity using a novel three-dimensional tissue culture model. Radiat Res. 1999;152:88–97. [PMC free article] [PubMed] [Google Scholar]
- 33.Azzam EI, de Toledo SM, Little JB. Direct evidence for the participation of gap junction-mediated intercellular communication in the transmission of damage signals from alpha -particle irradiated to nonirradiated cells. Proc Natl Acad Sci U S A. 2001;98:473–8. doi: 10.1073/pnas.011417098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mothersill C, Seymour C. Medium from irradiated human epithelial cells but not human fibroblasts reduces the clonogenic survival of unirradiated cells. Int J Radiat Biol. 1997;71:421–7. doi: 10.1080/095530097144030. [DOI] [PubMed] [Google Scholar]
- 35.Belyakov OV, Malcolmson AM, Folkard M, et al. Direct evidence for a bystander effect of ionizing radiation in primary human fibroblasts. Br J Cancer. 2001;84:674–9. doi: 10.1054/bjoc.2000.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huo L, Nagasawa H, Little JB. HPRT mutants induced in bystander cells by very low fluences of alpha particles result primarily from point mutations. Radiat Res. 2001;156:521–5. doi: 10.1667/0033-7587(2001)156[0521:hmiibc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 37.Zhou H, Suzuki M, Randers-Pehrson G, et al. Radiation risk to low fluences of alpha particles may be greater than we thought. Proc Natl Acad Sci U S A. 2001;98:14410–5. doi: 10.1073/pnas.251524798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.el-Fouly MH, Trosko JE, Chang CC. Scrape-loading and dye transfer. A rapid and simple technique to study gap junctional intercellular communication. Exp Cell Res. 1987;168:422–30. doi: 10.1016/0014-4827(87)90014-0. [DOI] [PubMed] [Google Scholar]
- 39.Yancey SB, Edens JE, Trosko JE, et al. Decreased incidence of gap junctions between Chinese hamster V-79 cells upon exposure to the tumor promoter 12-O-tetradecanoyl phorbol-13-acetate. Exp Cell Res. 1982;139:329–40. doi: 10.1016/0014-4827(82)90257-9. [DOI] [PubMed] [Google Scholar]
- 40.Matsumoto H, Hayashi S, Hatashita M, et al. Induction of radioresistance to accelerated carbon-ion beams in recipient cells by nitric oxide excreted from irradiated donor cells of human glioblastoma. Int J Radiat Biol. 2000;76:1649–57. doi: 10.1080/09553000050201145. [DOI] [PubMed] [Google Scholar]
- 41.Matsumoto H, Hayashi S, Hatashita M, et al. Induction of radioresistance by a nitric oxide-mediated bystander effect. Radiat Res. 2001;155:387–96. doi: 10.1667/0033-7587(2001)155[0387:iorban]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 42.Matsumoto H, Tomita M, Otsuka K, et al. A new paradigm in radioadaptive response developing from microbeam research. J Radiat Res. 2009;50(Suppl A):A67–79. doi: 10.1269/jrr.09003s. [DOI] [PubMed] [Google Scholar]
- 43.Matsumoto H, Tomita M, Otsuka K, et al. Nitric oxide is a key molecule serving as a bridge between radiation-induced bystander and adaptive responses. Curr Mol Pharmacol. 2011;4:126–34. doi: 10.2174/1874467211104020126. [DOI] [PubMed] [Google Scholar]
- 44.Shao C, Aoki M, Furusawa Y. Medium-mediated bystander effects on HSG cells co-cultivated with cells irradiated by X-rays or a 290 MeV/u carbon beam. J Radiat Res. 2001;42:305–16. doi: 10.1269/jrr.42.305. [DOI] [PubMed] [Google Scholar]
- 45.Shao C, Furusawa Y, Aoki M, et al. Nitric oxide-mediated bystander effect induced by heavy-ions in human salivary gland tumour cells. Int J Radiat Biol. 2002;78:837–44. doi: 10.1080/09553000210149786. [DOI] [PubMed] [Google Scholar]
- 46.Lehnert BE, Goodwin EH, Deshpande A. Extracellular factor(s) following exposure to alpha particles can cause sister chromatid exchanges in normal human cells. Cancer Res. 1997;57:2164–71. [PubMed] [Google Scholar]
- 47.Iyer R, Lehnert BE, Svensson R. Factors underlying the cell growth-related bystander responses to alpha particles. Cancer Res. 2000;60:1290–8. [PubMed] [Google Scholar]
- 48.Barcellos-Hoff MH, Brooks AL. Extracellular signaling through the microenvironment: a hypothesis relating carcinogenesis, bystander effects, and genomic instability. Radiat Res. 2001;156:618–27. doi: 10.1667/0033-7587(2001)156[0618:esttma]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 49.Prise KM, Folkard M, Michael BD. Bystander responses induced by low LET radiation. Oncogene. 2003;22:7043–9. doi: 10.1038/sj.onc.1206991. [DOI] [PubMed] [Google Scholar]
- 50.Egashira A, Yamauchi K, Yoshiyama K, et al. Mutational specificity of mice defective in the MTH1 and/or the MSH2 genes. DNA Repair (Amst) 2002;1:881–93. doi: 10.1016/s1568-7864(02)00113-1. [DOI] [PubMed] [Google Scholar]
- 51.Belyakov OV, Folkard M, Mothersill C, et al. Bystander-induced apoptosis and premature differentiation in primary urothelial explants after charged particle microbeam irradiation. Radiat Prot Dosimetry. 2002;99:249–51. doi: 10.1093/oxfordjournals.rpd.a006775. [DOI] [PubMed] [Google Scholar]
- 52.Iyer R, Lehnert BE. Low dose, low-LET ionizing radiation-induced radioadaptation and associated early responses in unirradiated cells. Mutat Res. 2002;503:1–9. doi: 10.1016/s0027-5107(02)00068-4. [DOI] [PubMed] [Google Scholar]