Abstract
Mapping chemical dynamics in the brain of live subjects is a challenging but highly rewarding goal because it allows neurotransmitter fluctuations to be related to behavior, drug effects, and disease states. A popular method for such measurements is microdialysis sampling coupled to analytical measurements. This method has become well-established for monitoring low molecular weight neurotransmitters, metabolites, and drugs, especially in pharmacological and pharmacokinetic studies. Recent technological developments which improve the temporal and spatial resolution of the methods will enable it to be used for studying behavior and small brain nuclei. Better assays allow monitoring more neurotransmitters simultaneously. Extension to analysis of aggregating proteins like amyloid β are proving extremely useful for uncovering the roles of these molecules and how they contribute to neurodegenerative diseases.
Introduction
Understanding the brain is one of the grand challenges facing science. Basic studies of brain function also have tremendous health and economic consequences as mental and neurological diseases such as Alzheimer’s, Parkinson’s, and depression are frequently long term, debilitating, and costly to treat resulting in significant loss of human potential and economic drain. In this review we consider progress made in monitoring neurochemicals in live animals as a method to study the brain. Neurons communicate by releasing neurotransmitters which diffuse across the synaptic cleft to interact with receptors on post-synaptic neurons. Besides this classical point-to-point communication, neurons may communicate by “volume transmission” wherein they release neurotransmitter or neuromodulator that diffuses beyond the synapse to affect neighboring neurons [1–2]. Measuring signaling chemicals and metabolites in the extracellular space can provide important insight into this chemical communication. By making such measurements in awake subjects, it is possible to correlate the chemical dynamics with behavior, disease progression, and drug effects in intact circuitry.
Tremendous challenges confront the scientist attempting in vivo neurochemical measurements. Neurotransmitters may change concentration rapidly requiring high temporal resolution. In some cases, one would like to follow changes that develop slowly over time, such as in the progression of drug dependency or a neurodegenerative disease, requiring great stability in measurements. Brain extracellular space is a soup of neurotransmitters, metabolites, energy molecules, and proteins so the chemical complexity of samples is high. Spatial compartmentalization of concentrations creates a challenge of spatial resolution as well. Measurement of dynamics within individual synaptic clefts represents the most extreme challenge. At a more macroscale level, the measurement within specific brain nuclei and sub-nuclei are important as such structures can be activated independent of neighboring regions and control specific behaviors or functions. Chemical release within sub-regions of brain nuclei < 1 mm3 can have identifiable functions [3–4]. A practical challenge is that for most experiments involving animal subjects, it is desirable to allow movement to observe behavior during the measurement. Therefore analytical measurements must be compatible with “samples on legs”.
Several techniques have been developed that allow in vivo neurotransmitter measurement including positron emission tomography (for reviews see [5–8]), cell based sensors [9], fluorescent tracers [10], implantable electrochemical sensors [11–15], and microdialysis sampling [16–17]. Although exciting advances have been made in all these areas recently, we will restrict our review to microdialysis sampling. In this approach, samples are collected from the brain extracellular space using a microdialysis sampling probe and resulting fractions analyzed for neurochemicals of interest. Traditionally this method has lower temporal resolution than the best sensors and lower spatial resolution because of the probe sizes. On the other hand, decoupling of sampling and detection allows the method to be extremely versatile. Thus, any analytical method can be coupled with the sampling probe to achieve the necessary sensitivity and selectivity for measurement. Methods may also allow multiple components to be determined in one fraction to study interactions among neurotransmitter systems. This method has been used for decades and is well established for pharmacological and pharmacokinetic studies. While many important neurochemical studies have used microdialysis in the past few years, in this review we will focus on recent technical advances and new applications for this approach to in vivo neurochemical monitoring. Although is microdialysis sampling is increasingly used for clinical studies [18–20], in this review we focus on advances related to fundamental neuroscience.
Improvements in Sampling: Large Molecules
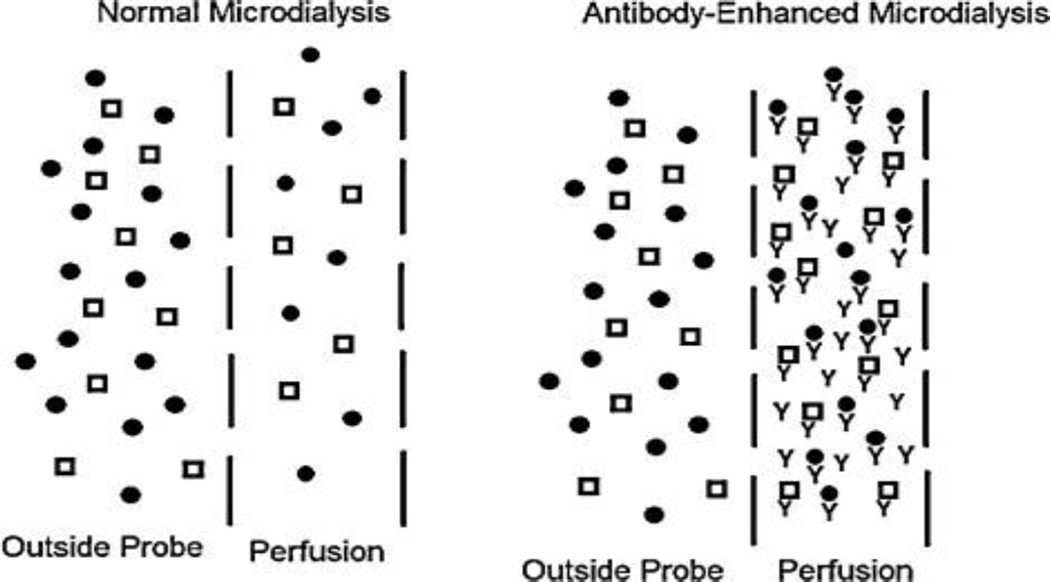
In microdialysis, a semi-permeable membrane is used as the probe. Substances in the extracellular space can cross the membrane according to their concentration gradient. Perfusion of the interior space both maintains the concentration gradient and drives the collected sample out of the brain for collection and analysis. A particular advantage of microdialysis is the ability to detect larger molecules (peptides and proteins) in the extracellular space that might be difficult to detect at conventional sensors. Many peptides, including both neuropeptides and other signaling molecules like cytokines, are present at low concentrations making their collection and assay difficult. An improvement in sampling is to add antibodies or other affinity agents to the perfusion flow to enhance the concentration gradient and recovery of the probes (see Figure 1) [21–25]. This approach has been used to enhance recovery several fold for neuropeptides and cytokines.
Figure 1.
Illustration of using antibodies to enhance recovery in microdialysis. In normal microdialysis, analytes (represented by shapes) cross the membrane according to their concentration gradient. In “antibody-enhanced” microdialysis, antibody added to the perfusion fluid (“y”-shapes) results in low concentration of free analyte in the dialysate. This maintains a high concentration gradient of free analytes resulting in higher recovered total concentration. (Adapted from [23].)
Measuring even larger proteins requires membranes that have high molecular weight cut-offs. Although it has been known for some time that large proteins can be sampled using microdialysis membranes with high molecular weight cut-off; this approach has been taken in an exciting direction by sampling proteins like amyloid-β (Aβ) [26–30] and tau [31]. After the initial validation of this method (see Figure 2), it has been used extensively to show that these proteins, which are known to form toxic plaques associated with Alzheimer’s disease, are released by neuronal activity in a way that explains regional vulnerability to plaque formation [26,28]. This important discovery has led to several insights about the pathology of Alzheimer’s and the role of these proteins in normal function. Similar studies of sampling of α-synuclein [32], the aggregating protein associated with Parkinson’s disease, have been reported suggesting that microdialysis will be a generally useful tool for studying this family of diseases and these proteins.
Figure 2.
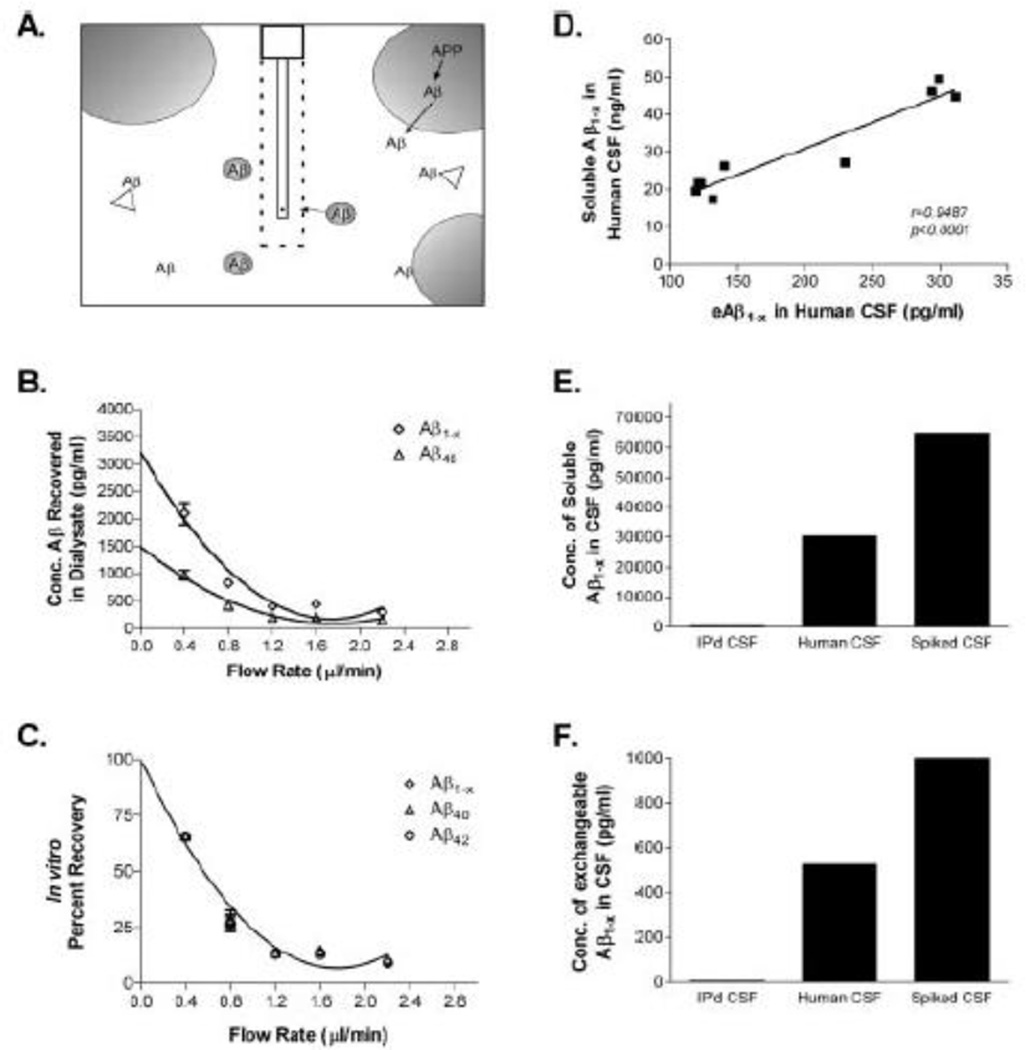
In vitro validation of method to measure free amyloid β (Aβ) in the extracellular space of a living brain by microdialysis. (A) Diagram of exchangeable Aβ. Triangles represent potential Aβ binding molecules, e.g., apoE, clusterin, and α2M. Only highlighted Aβ molecules are of the appropriate size to pass through a 35 kDA MWCO membrane on the microdialysis probe. (B) Interpolated zero flow method to quantify the pool of measurable Aβ1-x and Aβ40 within samples of human CSF (n = 4). At 2.2 µl/min, the percentage recovery of eAβ was 9.74 ± 1.53% (mean ± SEM). C, In vitro percentage recoveries for each Aβ species using the interpolated zero flow method. Each recovery point contains error bars and are overlapping for each species (n = 4). In vitro recovery of each Aβ species by microdialysis is the same. (D) The concentration of eAβ and total soluble Aβ are highly correlated within a sample of human CSF (Pearson’s r = 0.9487; p < 0.0001; n = 9). The mean concentrations of soluble Aβ 1-x and e Aβ 1-x were 30.47 ± 4.23 ng/ml (mean_SEM) and 196.2 ± 28.65pg/ml, respectively. (E,F) Human CSF immunoprecipitated (IP’d) for Aβ has undetectable levels of total soluble Aβ and eAβ. Human CSF spiked with an amount of exogenous Aβ40 peptide expected to double Aβ concentration resulted in a 2.1-fold increase in total soluble Aβ and a 1.9-fold increase in eAβ. (Figure and legend adapted from [27].)
Temporal Resolution
To a first approximation, temporal resolution of microdialysis is limited by the mass sensitivity of the analytical method used for assay. That is, enough sample must be collected per fraction to be able to detect the compounds of interest. As shown several years ago, highly sensitive methods like capillary electrophoresis with laser-induced fluorescence detection allow assay of fractions collected every few seconds to dramatically improve the temporal resolution over the minutes required when using HPLC for analysis [33–35]. The high speed of CE separations also made it compatible with the large number of samples generated by collecting fractions at seconds intervals. Work with such methods revealed that further improvements in temporal resolution were limited by flow and diffusion broadening of concentration zones as they were transported from the probe to the analytical system. While this broadening could be limited by using short connecting tubing, this approach was impractical for experiments with freely moving animals. This problem was solved by segmenting the outflow of a dialysis probe into droplets separated by an immiscible fluid (fluorinated oil) [36–37] (see Figure 3A). Segmentation was performed using microfluidic tee that could be mounted on the head of the animal to create a nanoliter volume fraction collector. Segmented samples do not mix by flow or diffusion and therefore retain the temporal information down to a few seconds regardless of the length of connection tubing needed. The limit on temporal resolution with such a system is broadening within the probe itself.
Figure 3.
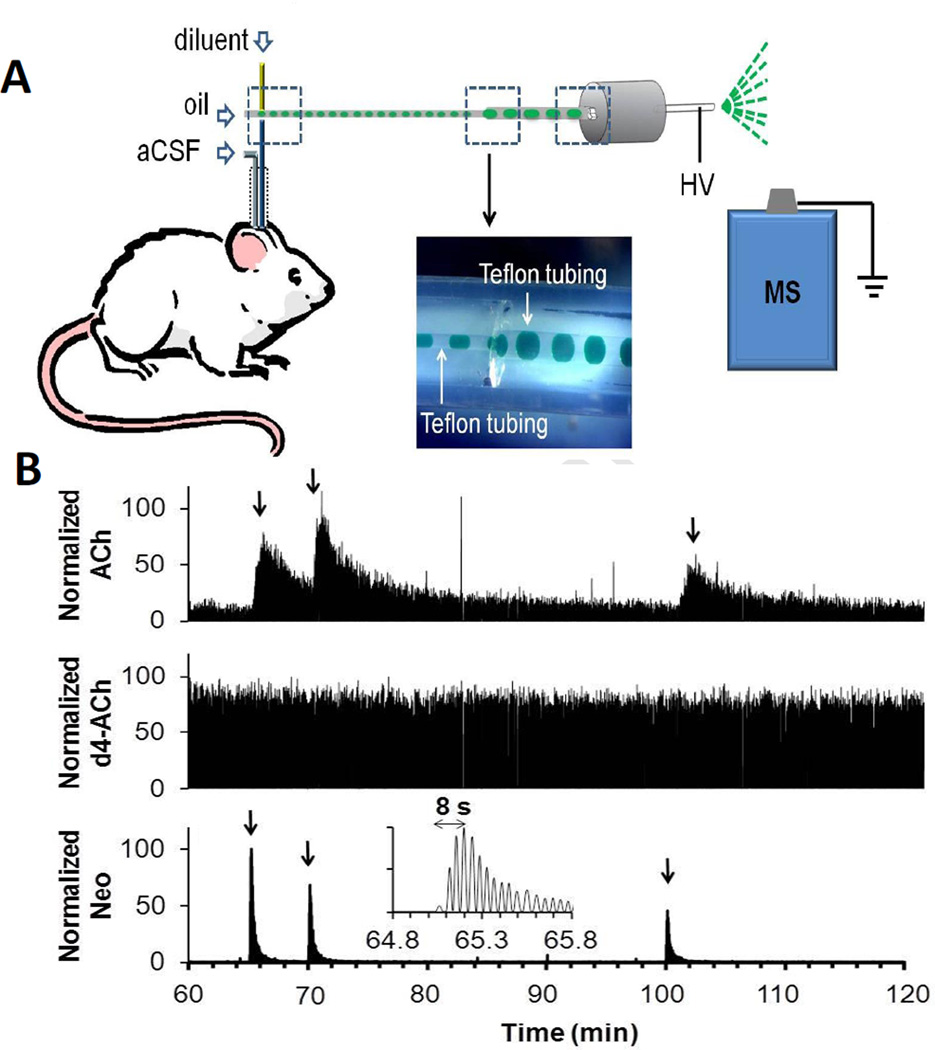
Illustration of segmented flow sampling and coupling to mass spectrometry. (A) Dialysate is pumped into a cross fluidic structure mounted on the subject’s head. In the cross, fluorinated oil and internal standard solution are pumped into separate arms. Resulting segmented flow emerges from the fourth arm and is pumped to the electrospray source of the mass spectrometer (MS). Inset shows a photo where aqueous solution of green dye was sampled and segmented within the cross by perfluorodecalin. HV stands for high voltage. (B) Recording of response to in vivo microinjection of neostigmine obtained using system in A with probe inserted in the striatum of a rat. Figure shows a sample trace for simultaneous detection of acetylcholine (ACh), internal standard, d4-acetylcholine (d4-ACh), and (C) neostigmine (Neo). Each compound is recorded at a specific m/z by the mass spectrometer. Arrow indicates beginning of each microinjection. Insert is a magnified view of the 1st microinjection for neostigmine detection showing the detection of individual droplets and rapid response time possible. (Adapted from [42]).
Microchip electrophoresis (amino acids) [38–39], enzyme assay (glutamate) [40], electrochemistry [41], and direct infusion mass spectrometry (acetylcholine) [42] (see Figure 3B) have been coupled to segmented flow systems to create high throughput assays of samples collected at a few seconds intervals. The mass spectrometry work is particularly interesting because it generates a “sensor” with the selectivity, multi-channel detection, and convenience of mass spectrometry detection. So far the segmented flow MS method has been used for acetylcholine. A challenge is to determine if conditions can be developed to allow direct MS analysis of other neurotransmitters by this approach. While most of the droplet work has been done with on-line detection, it is also feasible to collect and store droplets for later analysis [43].
Spatial Resolution
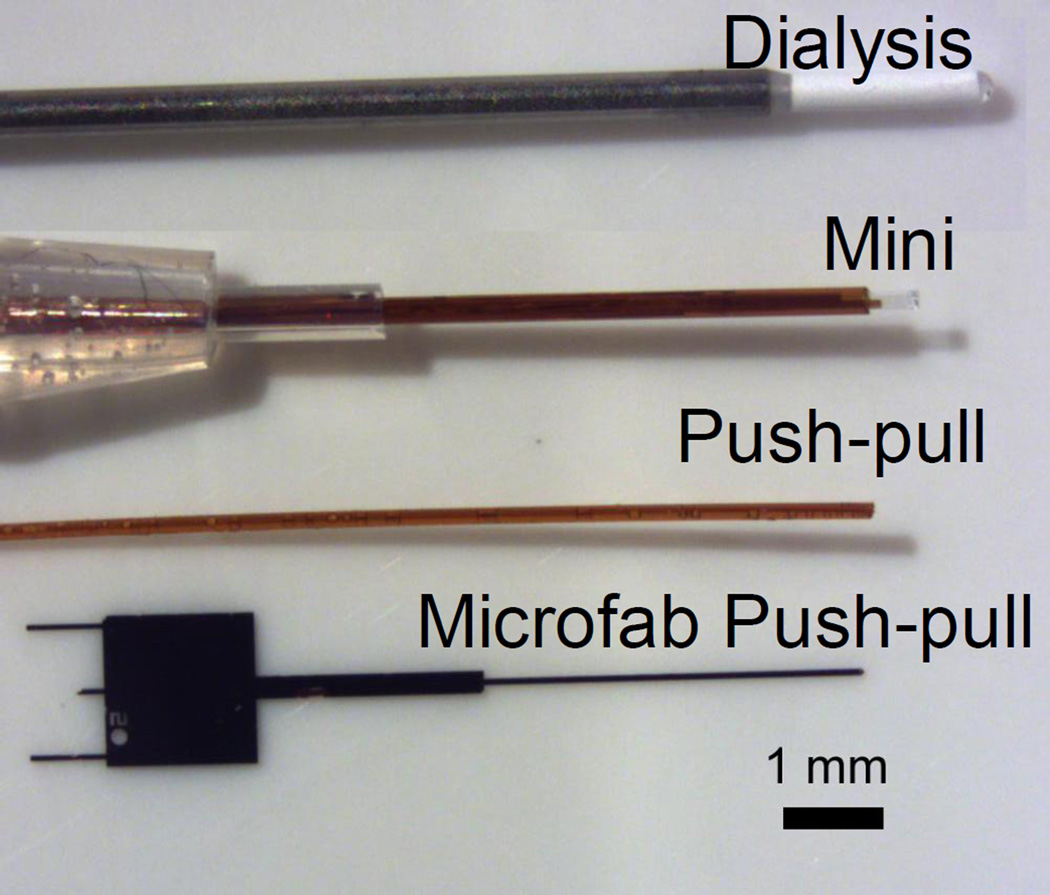
Microdialysis probes are usually 200 to 400 um diameter with a 1–4 mm sampling length. This size limits the potential to sampling from smaller brain regions. Smaller probes can be made (e.g. 0.5 mm long by 200 um diameter) and successfully used if the analytical method used is sensitive enough (Figure 4). A more radical approach is use of low-flow push-pull perfusion [44]. In this method, sampling occurs at the tip of two closely spaced capillaries. One fluid withdraws sample (“pull”) while make-up fluid is pumped from the other capillary (“push”) to maintain fluid balance in the sampling region. Sampling occurs just at the tip so spatial resolution is enhanced (Figure 4). Flow rates of 50 nL/min or less are used to minimize the potentially damaging effects of having direct contact of fluid with tissue. A limitation of these probes is poor temporal resolution. Consider that at 50 nL/min, it can take 20 min just to collect 1 µL for assay. Direct coupling to capillary electrophoresis can alleviate this concern [45]. Our group coupled the method with segmented flow to show that both high temporal and spatial resolution can be obtained [40]. In vitro results suggested sub-second sampling was possible creating the exciting prospect of combining the advantages of sampling methods (wide choice of analytical methods) with sensors (high spatial and temporal resolution).
Figure 4.
Comparison of the size of different sampling probes. Top probe is a conventional microdialysis sampling probe from a commercial vendor. White material is the sampling membrane. “Mini” is a small microdialysis probe that can be fabricated manually. The sampling area is the clear area (about 0.5 mm long by 0.2 mm diameter) at the tip. “Push-pull” is two fused silica capillaries, coated in polyimide, fused together with epoxy. Sampling occurs just at the tip. In practice, a rigid sheath if often added to support the fused silica to near the tip. “Microfab push-pull” a Si-based probe made by lithography and bulk etching techniques. The probe is 85 um wide by 70 thick along the shank and tip. Sampling occurs from a 20 um diameter orifice at the tip (not visible in the photo).
Although this approach is still in early developmental stage, initial applications have revealed encouraging results that highlight the spatial resolution [46–48]. For example, the method was coupled with LC-MS to show sharp gradients in basal concentration of several neurotransmitters even within small brain regions such as the ventral tegmental area [49]. This was the first direct detection of concentration gradients maintained across such small brain nuclei. The probes were estimated to sample from 4 nL voxels. The method was also used in the eye demonstrating potential beyond the CNS measurements. [46]
So far all push-pull probes have been assembled by hand from capillary tubes. We have recently developed an approach to microfabricating sampling probes in Si based on “buried channel technology” [50] (Figure 4). These probes have been successfully used in vivo. Microfabricated probes are much smaller than other sampling probes creating even better spatial resolution. Further, they offer the potential of mass fabrication, incorporating electrodes, and adding sample preparation steps into the probe.
Assay Methods
As discussed above, improvements in sampling were by necessity coupled to improved assay methods. Assays commonly used for microdialysis samples have been adapted to droplet methods and to the smaller probes as discussed above. Sensitive immunoassays were developed for measuring the larger proteins such as Aβ and tau. Many other clever assays have been developed for different neurotransmitters and coupled to sampling probes (see e.g. [51]). Besides these advances, a trend has been use of LC-MS for analyzing dialysate. For years HPLC with electrochemical or fluorescence detection was the method of choice for analyzing dialysate fractions; however, this method could only allow a sub-set of neurotransmitters to be analyzed in one fraction. Thus, easily oxidized neurotransmitters like dopamine and serotonin could be detected by LC-EC and amino acids were typically detected using fluorescence assays. Several groups have recognized the power of LC-MS and developed assays that allow different groups of neurotransmitters to be measured in one assay [52–57]. Perhaps the most comprehensive method is one that allows all the amino acid neurotransmitters, dopamine, serotonin, norepinephrine, adenosine, acetylcholine, carnosine, and many metabolites to measured in 8 min [58]. This approach relies on benzoyl chloride to derivatize the amine and phenol containing molecules making them easily retained on reversed phase LC and improving their ionization efficiency. Acetylcholine is not derivatized but can still be detected in the same assay. Use of MS greatly relaxes the separation requirements compared to EC or fluorescence detection since the method adds selectivity to detection. This method was shown to be useful for 1 min fractions.
For several years many groups have been developing capillary LC coupled to MS detecting neuropeptides in dialysate [59–67]. Much work over the past few years have been primarily demonstration and validation of detection of different neuropeptides. While very challenging because of the low concentration and instability of neuropeptide samples, this development is starting to bear fruit. For example, for the first time peptides were measured during a spontaneous behavior (feeding) [68]. This study revealed that enkephalins but not dynorphins increased in the dorsal striatum during feeding behavior. Microinjection of opioid agonist into the same brain region evoked feeding in sated rats showing that the opioid peptides were a signal to start eating. These results show the potential of the LC-MS methods to begin unraveling the role of neuropeptides in behavior.
Conclusions
In vivo sampling methods have maintained a stable popularity for studying neuropharmacology, pharmacokinetics and in measurement of relatively slow changes. Expansion to important larger molecules and improvements in temporal and spatial resolution are yielding renewed interest in these methods.
Highlights.
Enhanced recovery sampling allows trace level substances to be monitored in brain
Amyloid-β can be monitored to reveal its dynamics in plaque formation
Temporal resolution of 2 s for neurotransmitter monitoring can be achieved
Spatial resolution of 4 nL has been achieved with miniaturized sampling probes
LC-MS allows comprehensive monitoring of small molecule neurotransmitters
Acknowledgements
Our work in this area was supported by the McKnight Foundation and NIH R37 EB003320.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Agnati LF, Guidolin D, Guescini M, Genedani S, Fuxe K. Understanding wiring and volume transmission. Brain Research Reviews. 2010;64:137–159. doi: 10.1016/j.brainresrev.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Zoli M, Torri C, Ferrari R, Jansson A, Zini I, Fuxe K, Agnati LF. The emergence of the volume transmission concept. Brain Research Reviews. 1998;26:136–147. doi: 10.1016/s0165-0173(97)00048-9. [DOI] [PubMed] [Google Scholar]
- 3.Pecina S, Smith KS, Berridge KC. Hedonic hot spots in the brain. Neuroscientist. 2006;12:500–511. doi: 10.1177/1073858406293154. [DOI] [PubMed] [Google Scholar]
- 4.Smith KS, Berridge KC. Opioid limbic circuit for reward: Interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. Journal of Neuroscience. 2007;27:1594–1605. doi: 10.1523/JNEUROSCI.4205-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silverman DHS, Phelps ME. Application of positron emission tomography for evaluation of metabolism and blood flow in human brain: Normal development, aging, dementia, and stroke. Molecular Genetics and Metabolism. 2001;74:128–138. doi: 10.1006/mgme.2001.3236. [DOI] [PubMed] [Google Scholar]
- 6.Hartvig P, Bergstrom M, Antoni G, Langstrom B. Positron emission tomography and brain monoamine neurotransmission - Entries for study of drug interactions. Current Pharmaceutical Design. 2002;8:1417–1434. doi: 10.2174/1381612023394458. [DOI] [PubMed] [Google Scholar]
- 7.Lomena F. Non-oncological positron emission tomography (PET): Brain imaging. Medecine Nucleaire-Imagerie Fonctionnelle Et Metabolique. 2008;32:502–510. [Google Scholar]
- 8.Seneca N. Recent Advances in Positron Emission Tomography Imaging of Brain. Drugs of the Future. 2011;36:601–613. [Google Scholar]
- 9.Nguyen QT, Schroeder LF, Mank M, Muller A, Taylor P, Griesbeck O, Kleinfeld D. An in vivo biosensor for neurotransmitter release and in situ receptor activity. Nature Neuroscience. 2010;13:127-U301. doi: 10.1038/nn.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez PC, Pereira DB, Borgkvist A, Wong MY, Barnard C, Sonders MS, Zhang H, Sames D, Sulzer D. Fluorescent dopamine tracer resolves individual dopaminergic synapses and their activity in the brain. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:870–875. doi: 10.1073/pnas.1213569110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kita JM, Wightman RM. Microelectrodes for studying neurobiology. Current Opinion in Chemical Biology. 2008;12:491–496. doi: 10.1016/j.cbpa.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hashemi P, Walsh PL, Guillot TS, Gras-Najjar J, Takmakov P, Crews FT, Wightman RM. Chronically Implanted, Nafion-Coated Ag/AgCl Reference Electrodes for Neurochemical Applications. ACS Chemical Neuroscience. 2011;2:658–666. doi: 10.1021/cn2000684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keithley RB, Wightman RM. Assessing Principal Component Regression Prediction of Neurochemicals Detected with Fast-Scan Cyclic Voltammetry. ACS Chemical Neuroscience. 2011;2:514–525. doi: 10.1021/cn200035u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kile BM, Walsh PL, McElligott ZA, Bucher ES, Guillot TS, Salahpour A, Caron MG, Wightman RM. Optimizing the Temporal Resolution of Fast-Scan Cyclic Voltammetry. ACS Chemical Neuroscience. 2012;3:285–292. doi: 10.1021/cn200119u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerhardt GA, Huettl P, Pomerleau F, Quintero G, Burmeister J IEEE. Intra-Operative Chemical Diagnostics in the Brain Using Enzyme-Based Ceramic Microelectrode Arrays. 2010 IEEE Sensors. 2010:2338–2341. [Google Scholar]
- 16.Darvesh AS, Carroll RT, Geldenhuys WJ, Gudelsky GA, Klein J, Meshul CK, Van der Schyf CJ. In vivo brain microdialysis: advances in neuropsychopharmacology and drug discovery. Expert Opinion on Drug Discovery. 2011;6:109–127. doi: 10.1517/17460441.2011.547189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watson CJ, Venton BJ, Kennedy RT. In vivo measurements of neurotransmitters by microdialysis sampling. Analytical Chemistry. 2006;78:1391–1399. doi: 10.1021/ac0693722. [DOI] [PubMed] [Google Scholar]
- 18.Timofeev I, Carpenter KLH, Nortje J, Al-Rawi PG, O'Connell MT, Czosnyka M, Smielewski P, Pickard JD, Menon DK, Kirkpatrick PJ, et al. Cerebral extracellular chemistry and outcome following traumatic brain injury: a microdialysis study of 223 patients. Brain. 2011;134:484–494. doi: 10.1093/brain/awq353. [DOI] [PubMed] [Google Scholar]
- 19.Bossers SM, de Boer RDH, Boer C, Peerdeman SM. The diagnostic accuracy of brain microdialysis during surgery: a qualitative systematic review. Acta Neurochirurgica. 2013;155:345–353. doi: 10.1007/s00701-012-1582-z. [DOI] [PubMed] [Google Scholar]
- 20.Helmy A, Hutchinson P. Is cerebral microdialysis a clinical tool? Acta Neurochirurgica. 2013;155:355–356. doi: 10.1007/s00701-012-1588-6. [DOI] [PubMed] [Google Scholar]
- 21.Duo J, Stenken JA. Heparin-immobilized microspheres for the capture of cytokines. Analytical and Bioanalytical Chemistry. 2011;399:773–782. doi: 10.1007/s00216-010-4170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duo J, Stenken JA. In vitro and in vivo affinity microdialysis sampling of cytokines using heparin-immobilized microspheres. Analytical and Bioanalytical Chemistry. 2011;399:783–793. doi: 10.1007/s00216-010-4333-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbaugh AW, Stenken JA. Antibody-enhanced microdialysis collection of CCL2 from rat brain. Journal of Neuroscience Methods. 2011;202:124–127. doi: 10.1016/j.jneumeth.2011.05.006. Authors illustrate the highly effective use of antibodies added to perfusate to increase recovery of a tracel level peptide by several fold.
- 24.Pettersson A, Markides K, Bergquist J. Enhanced microdialysis of neuropeptides. Acta Biochimica Polonica. 2001;48:1117–1120. [PubMed] [Google Scholar]
- 25.Pettersson A, Amirkhani A, Arvidsson B, Markides K, Bergquist J. A feasibility study of solid supported enhanced microdialysis. Analytical Chemistry. 2004;76:1678–1682. doi: 10.1021/ac035305l. [DOI] [PubMed] [Google Scholar]
- 26. Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichle ME, Lee JM, Holtzman DM. Neuronal activity regulates the regional vulnerability to amyloid-beta deposition. Nature Neuroscience. 2011;14:750-U353. doi: 10.1038/nn.2801. This paper shows that regions with high synaptic activity under certain conditions release the most amyloid beta into the brain interstial space. These same regions develop amyloid plagues to a greater degree.
- 27.Cirrito JR, May PC, O'Dell MA, Taylor JW, Parsadanian M, Cramer JW, Audia JE, Nissen JS, Bales KR, Paul SM, et al. In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-beta metabolism and half-life. Journal of Neuroscience. 2003;23:8844–8853. doi: 10.1523/JNEUROSCI.23-26-08844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 29.Hong SY, Quintero-Monzon O, Ostaszewski BL, Podlisny DR, Cavanaugh WT, Yang T, Holtzman DM, Cirrito JR, Selkoe DJ. Dynamic Analysis of Amyloid beta-Protein in Behaving Mice Reveals Opposing Changes in ISF versus Parenchymal A beta during Age-Related Plaque Formation. Journal of Neuroscience. 2011;31:15861–15869. doi: 10.1523/JNEUROSCI.3272-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwetye K, Cirrito J, Esparza TJ, Mac Donald C, Holtzman DM, Brody DL. Dynamic Measurement of Soluble Human AB in a Combined Microdialysis-Controlled Impact Cortical Impact Mouse Model: Implications for Human Studies. Journal of Neurotrauma. 2009;26:A54–A54. [Google Scholar]
- 31. Yamada K, Cirrito JR, Stewart FR, Jiang H, Finn MB, Holmes BB, Binder LI, Mandelkow EM, Diamond MI, Lee VMY, et al. In Vivo Microdialysis Reveals Age-Dependent Decrease of Brain Interstitial Fluid Tau Levels in P301S Human Tau Transgenic Mice. Journal of Neuroscience. 2011;31:13110–13117. doi: 10.1523/JNEUROSCI.2569-11.2011. This article shows of the the first measurements of tau in brain extracellular space.
- 32. Emmanouilidou E, Elenis D, Papasilekas T, Stranjalis G, Gerozissis K, Ioannou PC, Vekrellis K. Assessment of alpha-Synuclein Secretion in Mouse and Human Brain Parenchyma. Plos One. 2011;6 doi: 10.1371/journal.pone.0022225. This article provides the first demonstration that the aggregating protein in Parkinson's disease, α-synuclein, is released into the brain and can be measured by microdialysis sampling and sensitive ELISA.
- 33.Lada MW, Vickroy TW, Kennedy RT. High temporal resolution monitoring of glutamate and aspartate in vivo using microdialysis on-line with capillary electrophoresis with laser-induced fluorescence detection. Analytical Chemistry. 1997;69:4560–4565. doi: 10.1021/ac970518u. [DOI] [PubMed] [Google Scholar]
- 34.Bert L, Robert F, Denoroy L, Stoppini L, Renaud B. Enhanced temporal resolution for the microdialysis monitoring of catecholamines and excitatory amino acids using capillary electrophoresis with laser-induced fluorescence detection Analytical developments and in vitro validations. Journal of Chromatography A. 1996;755:99–111. doi: 10.1016/s0021-9673(96)00595-x. [DOI] [PubMed] [Google Scholar]
- 35.Hogan BL, Lunte SM, Stobaugh JF, Lunte CE. On-line coupling of in vivo microdialysis sampling with capillary electrophoresis. Anal Chem. 1994;66:596–602. doi: 10.1021/ac00077a004. [DOI] [PubMed] [Google Scholar]
- 36.Wang M, Roman GT, Schultz K, Jennings C, Kennedy RT. Improved temporal resolution for in vivo microdialysis by using segmented flow. Analytical Chemistry. 2008;80:5607–5615. doi: 10.1021/ac800622s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deeba S, Corcoles EP, Hanna BG, Pareskevas P, Aziz O, Boutelle MG, Darzi A. Use of rapid sampling microdialysis for intraoperative monitoring of bowel ischemia. Diseases of the Colon & Rectum. 2008;51:1408–1413. doi: 10.1007/s10350-008-9375-4. [DOI] [PubMed] [Google Scholar]
- 38.Wang M, Roman GT, Perry ML, Kennedy RT. Microfluidic chip for high efficiency electrophoretic analysis of segmented flow from a microdialysis probe and in vivo chemical monitoring. Analytical Chemistry. 2009;81:9072–9078. doi: 10.1021/ac901731v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang M, Slaney T, Mabrouk O, Kennedy RT. Collection of nanoliter microdialysate fractions in plugs for off-line in vivo chemical monitoring with up to 2 s temporal resolution. Journal of Neuroscience Methods. 2010;190:39–48. doi: 10.1016/j.jneumeth.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slaney TR, Nie J, Hershey ND, Thwar PK, Linderman J, Burns MA, Kennedy RT. Push-Pull Perfusion Sampling with Segmented Flow for High Temporal and Spatial Resolution in Vivo Chemical Monitoring. Analytical Chemistry. 2011;83:5207–5213. doi: 10.1021/ac2003938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rogers M, Leong C, Niu XZ, de Mello A, Parker KH, Boutelle MG. Optimisation of a microfluidic analysis chamber for the placement of microelectrodes. Physical Chemistry Chemical Physics. 2011;13:5298–5303. doi: 10.1039/c0cp02810j. This article is the first demonstration of coupling segmented flow with electrochemical detection. This system will have value for high temporal resolution monitoring in vivo.
- 42. Song P, Hershey ND, Mabrouk OS, Slaney TR, Kennedy RT. Mass Spectrometry "Sensor" for in Vivo Acetylcholine Monitoring. Analytical Chemistry. 2012;84:4659–4664. doi: 10.1021/ac301203m. This article shows high temporal resolution monitoring of acetylcholine by a combination of microdialysis sampling, segmented flow, and direct infusion mass spectrometry. The mass spectrometer also allowed simultaneous monitoring of drugs and metabolites involved in acetylcholine signaling.
- 43.Wang M, Hershey ND, Mabrouk OS, Kennedy RT. Collection, storage, and electrophoretic analysis of nanoliter microdialysis samples collected from awake animals in vivo. Analytical and Bioanalytical Chemistry. 2011;400:2013–2023. doi: 10.1007/s00216-011-4956-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kottegoda S, Shaik I, Shippy SA. Demonstration of low flow push-pull perfusion. Journal Neuroscience Methods. 2002;121:93–101. doi: 10.1016/s0165-0270(02)00245-5. [DOI] [PubMed] [Google Scholar]
- 45.Patterson EE, Pritchett JS, Shippy SA. High temporal resolution coupling of low-flow push-pull perfusion to capillary electrophoresis for ascorbate analysis at the rat vitreoretinal interface. Analyst. 2009;134:401–406. doi: 10.1039/b813887g. [DOI] [PubMed] [Google Scholar]
- 46.Thongkhao-On K, Kottegoda S, Pulido JS, Shippy SA. Determination of amino acids in rat vitreous perfusates by capillary electrophoresis. Electrophoresis. 2004;25:2978–2984. doi: 10.1002/elps.200405941. [DOI] [PubMed] [Google Scholar]
- 47.Pritchett JS, Pulido JS, Shippy SA. Measurement of region-specific nitrate levels of the posterior chamber of the rat eye using low-flow push-pull perfusion. Analytical Chemistry. 2008;80:5342–5349. doi: 10.1021/ac800238d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thongkhao-On K, Wirtshafter D, Shippy SA. Feeding specific glutamate surge in the rat lateral hypothalamus revealed by low-flow push-pull perfusion. Pharmacology Biochemistry and Behavior. 2008;89:591–597. doi: 10.1016/j.pbb.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Slaney TR, Mabrouk O, Porter-Stransky KA, Aragona BJ, Kennedy RT. Chemical gradients within brain extracellular space measured using low flow push-pull perfusion sampling in vivo. ACS Chemical Neuroscience. 2013 doi: 10.1021/cn300158p. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Boer MJ, Tjerkstra RW, Berenschot JW, Jansen HV, Burger CJ, Gardeniers JGE, Elwenspoek M, van den Berg A. Micromachining of buried micro channels in silicon. Journal of Microelectromechanical Systems. 2000;9:94–103. [Google Scholar]
- 51.Perry M, Li Q, Kennedy RT. Review of recent advances in analytical techniques for the determination of neurotransmitters. Analytica Chimica Acta. 2009;653:1–22. doi: 10.1016/j.aca.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buck K, Voehringer P, Ferger B. Rapid analysis of GABA and glutamate in microdialysis samples using high performance liquid chromatography and tandem mass spectrometry. Journal of Neuroscience Methods. 2009;182:78–84. doi: 10.1016/j.jneumeth.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 53.Carrozzo MM, Cannazza G, Pinetti D, Di Viesti V, Battisti U, Braghiroli D, Parenti C, Baraldi M. Quantitative analysis of acetylcholine in rat brain microdialysates by liquid chromatography coupled with electrospray ionization tandem mass spectrometry. Journal of Neuroscience Methods. 2010;194:87–93. doi: 10.1016/j.jneumeth.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 54.Jerlhag E, Janson AC, Waters S, Engel JA. Concomitant Release of Ventral Tegmental Acetylcholine and Accumbal Dopamine by Ghrelin in Rats. Plos One. 2012;7 doi: 10.1371/journal.pone.0049557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Uutela P, Ketola RA, Piepponen P, Kostiainen R. Comparison of different amino acid derivatives and analysis of rat brain microdialysates by liquid chromatography tandem mass spectrometry. Analytica Chimica Acta. 2009;633:223–231. doi: 10.1016/j.aca.2008.11.055. [DOI] [PubMed] [Google Scholar]
- 56.Zhang XZ, Rauch A, Lee H, Xiao HB, Rainer G, Logothetis NK. Capillary hydrophilic interaction chromatography/mass spectrometry for simultaneous determination of multiple neurotransmitters in primate cerebral cortex. Rapid Communications in Mass Spectrometry. 2007;21:3621–3628. doi: 10.1002/rcm.3251. [DOI] [PubMed] [Google Scholar]
- 57.Gonzalez RR, Fernandez RF, Vidal JLM, Frenich AG, Perez MLG. Development and validation of an ultra-high performance liquid chromatography-tandem mass-spectrometry (UHPLC-MS/MS) method for the simultaneous determination of neurotransmitters in rat brain samples. Journal of Neuroscience Methods. 2011;198:187–194. doi: 10.1016/j.jneumeth.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 58.Song P, Mabrouk OS, Hershey ND, Kennedy RT. In Vivo Neurochemical Monitoring Using Benzoyl Chloride Derivatization and Liquid Chromatography-Mass Spectrometry. Analytical Chemistry. 2012;84:412–419. doi: 10.1021/ac202794q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Behrens HL, Chen RB, Li LJ. Combining microdialysis, nanoLC-MS, and MALDI-TOF/TOF to detect neuropeptides secreted in the crab, Cancer borealis. Analytical Chemistry. 2008;80:6949–6958. doi: 10.1021/ac800798h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lanckmans K, Sarre S, Smolders I, Michotte Y. Use of a structural analogue versus a stable isotope labeled internal standard for the quantification of angiotensin IV in rat brain dialysates using nano-liquid chromatography/tandem mass spectrometry. Rapid Communications in Mass Spectrometry. 2007;21:1187–1195. doi: 10.1002/rcm.2950. [DOI] [PubMed] [Google Scholar]
- 61.Lanckmans K, Sarre S, Smolders I, Michotte Y. Quantitative liquid chromatography/mass spectrometry for the analysis of microdialysates. Talanta. 2008;74:458–469. doi: 10.1016/j.talanta.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 62.Reed B, Bidlack JM, Chait BT, Kreek MJ. Extracellular biotransformation of beta-endorphin in rat striatum and cerebrospinal fluid. Journal of Neuroendocrinology. 2008;20:606–616. doi: 10.1111/j.1365-2826.2008.01705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang X, Rauch A, Xiao H, Rainer G, Logothetis NK. Mass spectrometry-based neurochemical analysis: perspectives for primate research. Expert Review of Proteomics. 2008;5:641–652. doi: 10.1586/14789450.5.5.641. [DOI] [PubMed] [Google Scholar]
- 64.Bernay B, Gaillard MC, Guryca V, Emadali A, Kuhn L, Bertrand A, Detraz I, Carcenac C, Savasta M, Brouillet E, et al. Discovering New Bioactive Neuropeptides in the Striatum Secretome Using in Vivo Microdialysis and Versatile Proteomics. Molecular & Cellular Proteomics. 2009;8:946–958. doi: 10.1074/mcp.M800501-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li Q, Zubieta JK, Kennedy RT. Practical Aspects of in Vivo Detection of Neuropeptides by Microdialysis Coupled Off-Line to Capillary LC with Multistage MS. Analytical Chemistry. 2009;81:2242–2250. doi: 10.1021/ac802391b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Van Eeckhaut A, Maes K, Aourz N, Smolders I, Michotte Y. The absolute quantification of endogenous levels of brain neuropeptides in vivo using LC-MS/MS. Bioanalysis. 2011;3:1271–1285. doi: 10.4155/bio.11.91. Illustration of the issues involved in validating neuropeptide measurments in vivo.
- 67.Mabrouk OS, Kennedy RT. Simultaneous oxytocin and arg-vasopressin measurements in microdialysates using capillary liquid chromatography-mass spectrometry. Journal of Neuroscience Methods. 2012;209:127–133. doi: 10.1016/j.jneumeth.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. DiFeliceantonio AG, Mabrouk OS, Kennedy RT, Berridge KC. Enkephalin Surges in Dorsal Neostriatum as a Signal to Eat. Current Biology. 2012;22:1918–1924. doi: 10.1016/j.cub.2012.08.014. This article describes the first detection of an opioid peptide during a spontaneous behavior. Microinjection studies showed that the release peptide was likely causing the behavior.