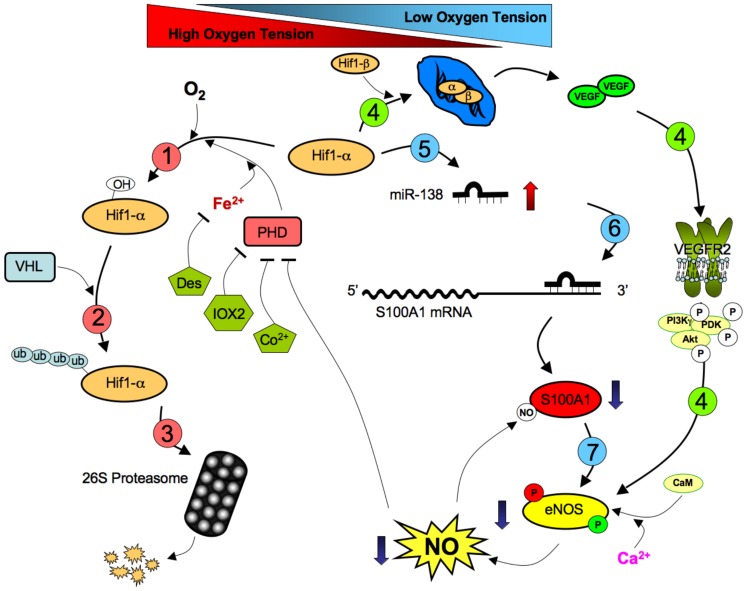
Figure 10. Proposed Scheme of S100A1 regulation by MiR-138.
(1) Under normal oxygen tension, the transcription factor Hif1-α is continuously hydroxylated by the action of cellular prolyl-hydroxylases (PHDs) in a reaction that requires Fe2+ and O2 as co-factors. (2) Hydroxylation of Hif1-α promotes binding of the Von Hippel-Lindau (VHL)-E3 ubiquitin ligase complex, promoting poly-ubiquitination and (3) degradation via the 26S proteasome complex. The action of PHDs are inhibited directly by IOX2 and Cobalt and indirectly by iron chelators, such as Desferroxamine (Des) as well as low oxygen levels. (4) Under low oxygen tension the Hif1-α protein becomes stabilized in the nucleus and promotes transcription of the pro-angiogenic vascular endothelial growth factor (VEGF) gene. VEGF promotes activation of eNOS by signaling through VEGFR2, promoting phosphorylation of the stimulatory Ser-1177 site. Increased eNOS activity raises nitric oxide (NO) production, which inhibits PHDs, further promoting Hif1-α stabilization in a positive feed-back loop. (5) To maintain cellular homeostasis, stabilization of Hif1-α also promotes increased production of MiR-138, (6) which binds to the 3′UTR of the S100A1 mRNA, leading to drastically reduced S100A1 levels and reduction of eNOS activity by promoting phosphorylation of the inhibitory Thr-495 site (7), in a counterbalancing negative feed-back loop. Endothelial dysfunction develops when these carefully balanced multiple feedback loops become dysregulated allowing for prolonged MiR-138 expression with consequent loss of S100A1 and reduced eNOS activity.