Abstract
Nanoparticles have emerged in the medical field as a technology well-suited for the diagnosis and treatment of a variety of disease states. They have been heralded as efficacious owing to both in terms of improved therapeutic efficacy as well as reduction of treatment side effects in some cases. Various nanomaterials have been developed which can be tagged with targeting moieties, and with drug delivery and imaging capability or combination of both as theranostic agent. These nanomaterials have been investigated for treatment and detection of various pathological conditions. The emphasis of this review is to demonstrate current research and clinical applications for nanoparticles in the diagnosis and treatment of renal diseases.
Keywords: nanoparticles, kidney disease, targeting, treatment, review
Introduction
The field of nanomedicine is rapidly progressing and widely expanding. Nanoparticles (NPs) have been investigated for numerous medical applications and are showing potential as a delivery method for diagnostic and therapeutic agents. Particularly, it is believed that NPs may be able to overcome certain biological and physical barriers where conventional therapies fail. NPs are colloidal dispersions consisting of an inner core and an outer shell, or a matrix structure that can encapsulate a drug, protein, imaging agent, or combination of therapeutic and imaging agents in a single nanostructure. Particle size is typically between 100 nm and 300 nm, although many formulations and applications call for smaller sizes. The agent of interest can be bound to the surface of NPs or encapsulated, allowing for delivery of therapeutics that would be otherwise unstable, insoluble, or biologically inactivated under typical conditions.
NPs for biological applications must be constructed in a way that they possess favorable characteristics when applied to cells, tissue, or serum. NPs inherently have a high surface area to volume ratio due to their small size. This results in a natural tendency to aggregate and interact with plasma proteins upon intravenous injection, leading to rapid clearance by the reticuloendothelial system (RES). Thus, NPs are often coated with hydrophilic and biocompatible polymers, such as polyethylene glycol (PEG) or pluronics, which protect against opsonization and endocytosis, and afford an extended plasma half-life 1–3. The manipulation of these coating materials and size of the NP in tern alters the drug release profile and drug concentration delivered.
Oral route of drug delivery is often hampered by absorption, local toxicity, enzymatic degradation and solubility in the gastrointestinal system. Polymeric NPs have been actively explored as oral delivery vehicles for pharmaceutically challenging molecules 4. It has been shown that NPs can improve the oral bioavailability of drugs that have poor water solubility and high molecular weight 5. This can be achieved by modifying both the physiochemical properties and by coupling targeting molecules to the NP surface 6. Hydrophilic polymers such as PEG and chitosan have been shown to enhance delivery across the intestinal mucosa 7,8. Proteins, vaccines, and nucleic acid material have shown promising results for oral delivery and all have potential applications to kidney disease.
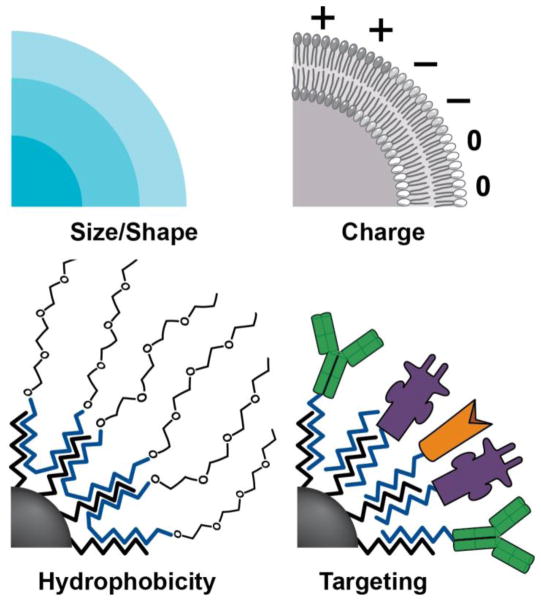
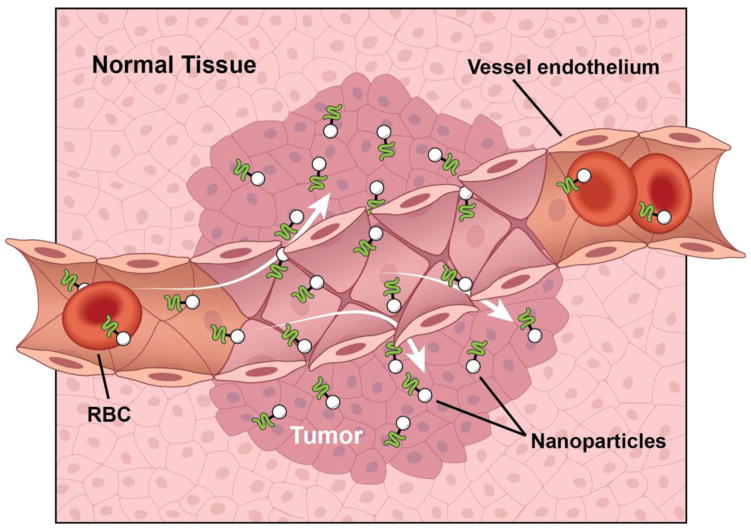
Along with their physicochemical properties helping NPs evade certain biological barriers, NPs surfaces can be manipulated to target end organs or tissues via the surface polymer charge, shape, or conjugated ligand. Methods have been developed to make NPs hydrophobic or create electrostatic charges, which improve sight-specific delivery 9 (Figure 1). NPs can be manipulated also by conjugation of specific ligands to the surface of the particle in the attempt to target a specific sight, including antibodies and peptides which target specific cell receptors. For example, tumor cells express various molecular markers not expressed on normal tissue, which can be used as docking sites to concentrate drug loaded NPs to tumors 10. Furthermore, the vasculature endothelium of tumor cells can be targeted to enhance NP delivery and take advantage of the tumor enhanced permeability and retention effect 11 (Figure 2). Table 1 summarizes the characteristics and targets of various NPs created and being studied for treatment of renal diseases.
Figure 1.
The properties of NPs are able to be modified by size, shape, charge, hydrophobicity, and with targeting agents such as amino acids, proteins or ligands.
Figure 2.
The enhanced permeability and retention effect of tumor vasculature and lymphatics. Leaky blood vessel endothelium allows escape of small particles such as NPs into the surrounding tumor. Poorly organized lymphatic channels increased retention of the NPs at the target tumor.
Table 1.
Characteristics of NPs with renal targeting
| Characteristics | Renal Target | |
|---|---|---|
| Size | 80–100 nm gold core NP | Mesangium |
| 4–6 nm iron oxide core NP | Cortex, nephritis | |
| Conjugation | MHC-II antibody | Medulla |
| Anti-CDIIb antibody | Unilateral obstruction | |
| Integrin ανβ3 | Tumor vasculature | |
| Encapsulation | PLGA coated CoQ10, superoxide scavenger | Vascular endothelium |
| Dendrimer | Medulla during ischemia | |
| Dextran coated iron oxide | Cortext during allograft rejection | |
| Peg coated nucleic acids | Cell nucleus, gene therapy | |
| TNF-α | Tumor interstitium |
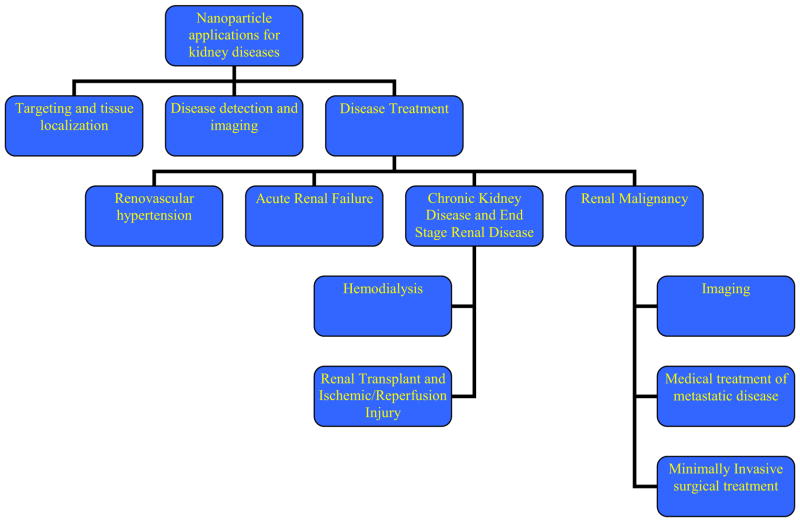
The vast range of diseases affecting the kidney often requires both a medical and a surgical treatment course. The diverse research and applications of NPs in the field of kidney disease alone has opened new avenues of treatment, disease detection, and disease monitoring. Problems are acute and chronic in nature and often involve a nephrologist and urologist. Here we explore the growing field of NPs in various kidney diseases and their experimental as well as clinical applications and why the practicing clinicians should have knowledge of this new technology (Figure 3).
Figure 3.
Schematic for different applications of Nanotechnology in kidney diseases.
NPs in Renal Targeting and Renal Imaging
The kidney is a unique organ for targeting of NPs due to its innate ability to rapidly clear particles that are smaller than 10 nm in diameter 12. This is accounted for by the glomerular filtration unit, the basement membrane, and the interdigitating podocytes. Within the renal corpuscle, there exists a fenestrated endothelium separating the mesangium from the extracellular matrix. Therefore, 2 nm particles are readily cleared by the kidney, but significantly decreased when size is 6 nm and virtually no renal excretion at 11 nm diameter 13.
Choi et al devised gold-loaded nanoparticles of serial core diameters entrapped by PEG polymer of various sizes 12. They demonstrated that NPs of 80–100 nm target the mesangium of the kidney, where smaller particles are seen only in the peritubular capillaries and the largest particles are taken up by the Kupffer cells in the RES of the liver and spleen.
Another method of NP targeting involves immunotargeting. NPs can be created such that functional groups on the exterior can be easily conjugated to a broad range of biological molecules such as proteins or peptides. NPs tailor made that encapsulate imaging contrast agents, such as iron oxide when deployed with MRI, make it possible to diagnose, track disease, track cells and tissues, and deliver of therapeutics 14–16. By combining these approaches of immunotargeting and encapsulation of a contrast agent, Hultman et al used major histocompatability complex (MHC) anti-MHC II antibodies as a marker of adaptive immune response and inflammation to target the renal medulla of the rat 3. This is thought to be more specific than simply targeting macrophages which nonspecifically accumulate at sites of inflammation. They show a five-fold longer half-life in the renal medulla specifically with MHC II antibody conjugated iron oxide NPs than non-conjugated NPs 3. The ability to target a specific compartment of the kidney has implications for delivery of therapeutics as well as monitoring disease states affecting different portions of the kidney.
The kidney has been targeted with magnetic NPs as well. Magnetic NPs work by applying an external magnet near the target region to localize the magnetic field 17. In a proof of concept study, Kumar et al demonstrated fluorescent magnetic NPs could be selectively delivered to the kidney in a mouse model more so than non-magnetized particles 18.
NPs for Renovascular Hypertension
Free radicals play a role in cardiovascular disease and antioxidants are being explored for their benefits in reducing the oxidative stress pathway 19. Agents targeted at free radical scavenging have not been previously used in treatment of cardiovascular disease due to poor physicochemical and biopharmaceutical properties leading to low oral bioavailabity 19. One such free radical scavenger, coenzyme Q10 (CoQ10), plays a critical role in cellular respiration in the mitochondria as well as functions as an antioxidant, free radical scavenger, and inhibitor of lipid peroxidation 20. CoQ10 has been found to be effective in cardiovascular disorders like cardiomyopathy, hypertension, angina pectoris and atherosclerosis, as well as cardiotoxicity caused by doxorubicin 21,22. Clinical trials have demonstrated the potential of CoQ10 in treatment of hypertension in humans by means of decreasing total peripheral resistance and therefore resulting in vascular wall relaxation 23. CoQ10 also acts as an antagonist for vascular superoxides, either scavenging them or suppressing their synthesis 24.
To overcome the hurdles of drug delivery of CoQ10, PLGA formulated NPs have been synthesized to increase oral bioavailability and establish a therapeutic effect of CoQ10 19. A Goldblatt 2 kidney 1 clip model was used for induction of hypertension in a rat model. CoQ10 encapsulated NPs were injected intravenously for 12 days and the experimental animals showed a significantly reduced blood pressure as compared to suspended CoQ10 and at 60% lower dose 19. This shows the efficacy of CoQ10-NPs at blood pressure reduction and potential as a therapeutic for humans.
Another area of research has examined the antihypertensive properties of cytochrome P450 metabolites. One such metabolite is epoxyeicosatrienoic acid, which has been shown to demonstrated vasodilatory properties in vascular beds independent of nitric oxide or prostaglandins 25. However, epoxyeicosatrienoic acid is readily cleaved to its less active diol form by epoxide hydrolase 26. 1,3-Dicyclohexyl urea (DCU) is a potent soluble epoxide hydrolase inhibitor and has been shown to lower systemic blood pressure but can only be given intraperitoneally in rat models due to its poor aqueous solubility 27. Ghosh et al sought to increase solubility and bioavailability by creating a DCU nanosuspension, which increased surface area 40-fold and enhanced oral bioavailability 28. Furthermore, they were able to show a significant difference in a hypertensive rat model in both angiotensin II levels as well as decreasing systolic pressures by nearly 30 mmHg 28. Improvement in drug oral bioavailabity would have wide application for treating patients on a chronic outpatient basis.
Atherosclerosis is closely associated with hypertension and the proportion of patients with lesions and hypertension is higher than those with normal blood pressure 29. The renin-angiotensin-aldosterone system (RAAS) plays an integral role in hypertension and is closely related to and affected by atherosclerosis. Angiotensinogen, a precursor to angiotensin I, has been found to be significantly elevated in hypertensive patients 30. Embracing these principles, Lu et al created nanoparticles loaded with silencing RNA (a short hairpin RNA) against angiotensinogen for the treatment of a hypertensive rat model 31. They found the mRNA expression of angiotensinogen in the liver of treated rats was markedly reduced compared to control groups and, furthermore, the systolic blood pressure was reduced by an average of 27 mmHg compared to pretreatment levels. Moreover, histological examination of major vessels indicated that the inflammatory changes associated with atherosclerotic plaques were attenuated after treatment as well 31.
NPs for Acute Renal Failure
Acute renal failure (ARF) is life-threatening, occurs in up to 20% of intensive care unit patients, and carries a high mortality rate 32. Early diagnosis and initiation of therapy is necessary for reducing morbidity and mortality. ARF has numerous causes including hypotension/sepsis, trauma, acute tubular necrosis (ATN), pharmaco-toxins causing acute interstitial nephritis (AIN), contrast injury, and urinary obstruction.
Current diagnosis of ARF requires serum blood tests and commonly examines blood urea nitrogen (BUN) and creatinine levels. Problems with these tests are numerous, including the non-specific/diagnostic nature of the tests and the delay in rise in these elements can be up to 24 hours 33. One method for detection of ARF that has been explored uses a dendrimer nanoparticle-based magnetic resonance imaging (MRI) technique 34,35. A dendrimer is a repetitively branched molecule that often takes the form of a sphere with symmetry established around a core with functional groups at the surface. An array of dendrimer nanoparticles were used to examine their ability to detect even short amounts of ischemia in a mouse model and found that the size of the poorly enhanced area of kidney correlated with the duration of ischemia and reperfusion time 34. Again, in a mouse model of sepsis induced ARF, it was shown that dendrimer-encapsulated contrast NPs coupled with MRI could detect renal injury before serum creatinine became elevated 35. This has implications for potential early institution of therapy or removal of nephrotoxic elements before kidney damaged is detectable in the serum.
In an animal model of acute tubular nephrotoxicity cause by cisplatin, MRI was used to study changes in medullary enhancement 34. It was observed that imaging with dendrimer-coated NPs was able to distinguish medullary enhancement loss after tubular injury when normal phase MRI could not. Signal loss on NP enhanced MRI was proportional to the degree of renal damage 34.
Differentiation of kidney disease can also be a diagnostic challenge, often relying on renal biopsy, which is invasive and has potential for complications. Macrophage activity in the normal state is virtually absent in the kidney, but it occurs frequently in nephritis, renal transplant rejection, and renal obstruction 36–38. Hauger et al sought to determine if macrophage activity could be imaged and localized to compartments of the kidney based on disease type. In a rat model, ultrasmall superparamagnetic iron oxide (USPIO) NPs 4–6 nm in diameter and coated with dextran were injected into a nephrotoxic nephritis model (anti-basement membrane, equivalent to Goodpasture syndrome) or into an obstructive nephropathy model, which induces diffuse interstitial lesions throughout the kidney. MRI signal intensity with the NPs loss localized in the cortex (where glomerular lesions occur) in the nephritis model and localized to the entire kidney in the obstructive model. Furthermore, the degree of signal intensity loss was correlated to the degree of proteinuria in the nephritis model.
Urinary or ureteral obstruction has been implicated in the processes of acute interstitial renal disease and inflammation. In an animal model, unilateral renal obstruction has been shown to cause an increase in the collagenous fibrous tissue in the renal interstitium after 6 days from time of injury 39. This effect was able to be visualized with the aid of silica loaded nanoparticles in the following model. Taking advantage of the inflammatory cascade, fluorescent anti-CD11b nanoprobes were designed (CD11b is expressed on the surface of mouse macrophages) and injected intravenously into a mouse model of unilateral ureteral obstruction. The NPs were accumulated in the UUO kidney to a significantly greater extent compared with the normal, non-inflamed kidney 39.
NPs for use in Renal Transplantation and Ischemic-Reperfusion Injury
Renal ischemic is a reduction of cortical blood flow causing tissue hypoperfusion that can be caused by disease states such as sepsis, hypotension, or renal artery stenosis (RAS) or iatrogenically such as after partial nephrectomy or during renal transplantation. After renal ischemia, renal blood flow returns toward normal but regional alterations occur. The outer medullary region is marginally oxygenated under normal conditions and has high energy demands, specifically the thick ascending limb of the renal tubule 40,41. The blood flow to this region after reperfusion amounts to ~10% of normal, leading to regional congestion due to edema, poor blood flow, deprivation of nutrients and loss of ATP 42. ATP degradation products such as hypoxanthine can leak out of cells and can be converted by xanthine oxidase to uric acid and form reactive oxygen species in the process, contributing to ischemic damage 41. Tubular cell swelling in the confined space of the outer medulla mechanically adds to capillary obstruction and causes medullary congestion, decreased medullary blood flow, and further perpetuating ischemia and tubular cell injury 41.
A recent study suggests that permanent damage to peritubular capillaries occurred in rats that underwent renal ischemia and may partly account for the pathogenesis of chronic renal failure in this setting 43. Renal ischemia leads to cell necrosis by generation of breakdown products of cell energy such as ROS, increase in free cytosolic calcium, and activation of phospholipases, proteases, and endonucleases 41,44. Cellular debris and intratubular protein from casts and obstruct the tubules, causing increased pressures proximal to the obstruction; the fluid that leaks out from the obstructed tubules causes tissue edema 41. The oxygen burst phenomenon is a trigger for complex biochemical reactions leading to generation of oxygenated lipids and changes in microcirculation with recruitment of neutrophils after reperfusion of a transplanted organ 45.
Agents such as mannitol that inhibit cell swelling partially protect the kidney against the functional deficits induced by ischemia and also decrease medullary congestion 46. Scavengers such as SOD, glutathione, and vitamin E, as well as inhibitors of ROS production, such as the iron chelators (e.g. deferoxamine) have been reported to protect against ischemic injury 47,48.
Two molecules that have been widely studied and implicated in ischemia-reperfusion injury include endothelin and nitric oxide, which regular vascular tone and may affect leukocyte adhesion 49. Nitric oxide may decrease adhesion of epithelial cells enhancing ischemia-reperfusion injury by increasing the detachment of tubule cells resulting in intraluminal obstruction 49. The inflammatory response may be implicated in renal injury as leukocytes infiltrate and cause edema by compromising microvascular blood flow 50. Tubule segments in the outer medulla, especially the proximal tubule, are most susceptible to injury given its limited capacity to undergo anaerobic metabolism in the setting of a decrease in oxygen tension 49.
ROS are toxic to tubular epithelial cells and scavengers such as superoxide dismutase, glutathione and vitamin E, and inhibitors of ROS production, such as desferoxamine, have been reported to protect against renal injury 47,48. Ischemia-reperfusion produces excessive ROS that are unable to be handled by the tissues normal scavenging system 51. Excessive ROS leads to cell damage by lipid peroxidation, DNA breakdown, and protein damage. ROS have been implicated as contributors to chronic allograft lose following transplantation due to development of renal fibrosis through loss of epithelial cell membrane transporters and their functions and the acquisition of a more fibroblastic phenotype leading to production of extracellular matrix elements such as collagen and fibronectin 51. Superoxide dismutase (SOD) appears to be the most important enzymes involved in the defense system against reactive oxygen species 6, particularly against superoxide anion radicals 52. Delivery of superoxide dismutase to the sites of potential free radical injury has been shown to reduce fibrosis in animal models 51.
The therapeutic utility of antioxidants is hindered by inadequate delivery due to short intravascular half life and susceptibility to proteolysis 53. NPs for delivery of SOD have been explored but not yet specifically in the kidney. Chen et al created silica NPs containing SOD and conjugated them to a transactivator protein (TAT) to enhance intracellular delivery 54. SOD NPs have been utilized in a stroke model as well 55. Yun et al showed that SOD loaded NPs reduced neuronal cell death in vitro and in a mouse model, SOD NPs reduced stroke volume by 50%, reduced inflammatory markers, and improved mouse neuronal function post-injury 55,56.
Monitoring renal allografts after transplantation is a necessary part of long term care. Specifically early detection of graft rejection and institution of therapy may provide increased longevity of graft survival and decrease morbidity of ESRD. Although numerous techniques have been used for the detection of graft rejection, development of specific, sensitive, and non-invasive methods for the diagnosis of rejection is still a major challenge in the field of renal transplantation57. Renal biopsy, the “gold standard” for rejection diagnosis, can be complicated by hemorrhage, infection, and arteriovenous fistula (AVF). MRI has the potential to be utilized for early detection of allograft rejection when coupled with dextran-coated superparamagnetic iron oxide nanoparticles 57,58. These particles have previously been utilized as a vascular contrast agent as well as to detect macrophage infiltration into the kidney 59–61. As renal allograft rejection is histologically marked by infiltration of T-cells and most strongly with persistence of macrophages, a method of monitoring this process would aid in the treatment of rejection. Ye et al was able to demonstrate in a rat model a predictable and significant difference between MR signal intensity in the renal cortex of renal grafts with iron oxide nanoparticles in allografts as compared to isografts 57. This could be a valuable and noninvasive method of detecting acute rejection.
Cyclosporine (CsA) is a first-line immunosuppressant used to prevent graft rejection and in treatment of autoimmune disorders. Until the recent production of the drug Neoral, CsA manufacturing has been associated with low or variable oral bioavailability 62. Nephrotoxicity is a significant challenge to overcome with new formulations of CsA. Efforts have been made to create nanoparticulate formulations of CsA to improve bioavailability as well as reduce toxicity 63,64. PLGA-full form NPs were prepared at different loading concentrations and tested in a rat model at different doses to examine toxicity and tissue levels for 30 days 19. It was shown that NP formulation at 30 mg/kg and 30% loading produced a similar maximal concentration of CsA with significantly lower nephrotoxicity. Additionally, they observed smaller fluctuations of blood CsA concentrations over 30 days than with Neoral due to the sustained NP release effect 19. Drug preparation for immunosuppresion will continue to be a major portion of renal transplant patient care.
NPs in Chronic Kidney Diseases
Chronic kidney disease (CKD) is a progressive loss of renal function over months to years marked by a loss of glomerular filtration capability. In CKD, toxins, which would otherwise be excreted by the normal functioning kidney, are able to accumulate in the blood. Following creatinine and BUN levels for CKD have the short-comings of varying with age, gender, diet, muscle mass, muscle metabolism, medications, diet and hydration status 65. However, much of kidney function can be lost before accumulation of toxins in the blood can be detected, leading to late diagnosis and treatment of CKD.
A novel method for identifying CKD and disease progression has been explored that utilizes breath testing of volatile organic compounds (VOCs) 66. Work by Haick et al demonstrated a non-invasive means of detecting end-stage renal disease (ESRD) in a rat model based on a carbon-nanotube-based sensor and breath sample analysis 67. The work was then continued with a focus on identification of early-stage CKD in which patients with CKD had breath analysis performed with gold nanoparticle sensors. The NP coated sensors acted to extract the VOCs from breath samples, which could then be analyzed by gas chromatography. The results showed that a combination of gold NP sensors could distinguish with high accuracy between the exhaled breath of early-stage CKD and healthy states, advanced and end-stage CKD, as well as ranges in between 68. This technology has the possibility to improve screening methods for CKD and early detection and treatment.
Magnetically assisted hemodialysis (MAHD) has been proposed as a more efficient means of management of ESRD. In MAHD, biocompatible ferromagnetic NPs are bound to targeted binding substances which have high affinity for serum toxins in the body. The binding substances proposed for utilization could be proteins, immunoglobulins, or antibodies. Body areas can then be targeted with magnetic manipulation of the iron component of the NPs. The proposed system would function by infusion of the NPs prior to HD session, thus, allowing adequate time for the binding on NPs to toxic substances prior to the actual HD session. The study evaluated the toxin homocysteine and demonstrated that it was readily absorbed onto the targeted carriers and then cleared rapidly in in vitro studies 69. Furthermore, they were able to demonstrate that after one circulation of blood volume, only an immeasurably small magnetic signal remained in the blood. This is compared to the required roughly 8 circulations of blood volume in current HD therapy. The implication is the MAHD would achieve improved clearance of toxins in a shorter period of time improving the quality of life of ESRD patients.
Nephrologists frequently treat anemia associated with chronic kidney disease. Anemia in CKD patients results from a lack of production of erythropoietin by the kidneys. Furthermore, CKD is an inflammatory condition leading to sequestration and impaired release of iron from macrophages of the RES in the liver, spleen, and bone marrow, or reticuloendothelial blockade 70. Hepcidin, a compound produced by the liver during inflammation, inhibits oral iron absorption in the small intestine and thus limits bioavailability of conventional oral therapy 71. Ferumoxytol is a superparamagnetic iron oxide nanoparticle that has a polyglucose carboxymethylether coating and a particle diameter of 30 nm and molecular weight of 731kD 72. There are several Phase III clinical studies regarding the treatment efficacy of ferumoxytol with patient both prior to initiation of HD and those receiving long-term HD which demonstrate that compared to oral iron therapy, ferumoxytol given intravenously causes a significantly greater percentage of patients to achieve a 1g/dl increase in hemoglobin blood values 73–75. With similar adverse events compared to oral therapy, the NP preparation of ferumoxytol has been shown to be an efficacious treatment for iron deficiency anemia associated with CKD.
In chronic inflammatory diseases of the kidney such as lupus nephritis, the degree of renal involvement varies and currently there exist no urine or serum biomarkers that predict biopsy results for severity of involvement 76,77. Examination of renal tissue for C3 fragment deposition within the flomeruli is a routine part of the evaluation of renal biopsies, and tissue C3 deposits are interpreted as evidence of complement activation 77. Imaging of antibodies in immune complexes relative to circulating antibodies is unlikely to be useful, given the small differences in antibody concentration between the blood pool and deposits when averaged over imaging voxels 76. However, targeting cleavage products of the complement system with nanoparticles enables the focus on tissue-bound fragments rather than circulating intact complement and provides a target for imagine. Serkova et al have developed an animal model of nephritis and used targeted magnetic resonance imaging with nanoparticles to monitor progression over time 78. Antibodies to the cleavage product of C3 (C3d) were encapsulated in magnetic NPs, allowing monitoring of changes in inflammation in the kidney by MRI 78. This type of non-invasive, organ specific monitoring potentially allows titratable treatment of disease to preserve renal function even prior to detection of renal deterioration by serum chemistry testing.
Half of all Americans have CKD over the age of 70 and nearly 25% of patients undergoing angiography studies have CKD 79. For these patients, iodinated contrast or gadolinium based contrast could mean worsening of disease to the point of needing renal replacement therapy, or could be fatal with gadolinium and nephrogenic systemic fibrosis (NSF) 1. Magnetic NP imaging is a safe modality for these situations and uses superparamagnetic iron oxide NPs to localize the spatial position of the NPs with nearly no background tissue noise 80. These iron oxide particles are processed and stored in the liver with the body’s iron reserve to be used in hemopoesis and do not affect the kidneys 81.
NPs for Neoplasms of the Kidney
Imaging
Metastatic renal cell carcinoma (RCC) is nearly always a fatal disease. These highly vascular tumors are chemo- and radio-resistant and thus current treatment regiments consist of vascular targeting agents, such as tyrosine kinase inhibitors (TKIs), vascular endothelial growth factor (VEGF) inhibitors and mammalian target of rapamycin (mTOR) inhibitors, which help slow disease progression. After institution of therapy, patients are followed clinically with axial imaging either computed tomography (CT) or MRI. Size of tumor serves as a surrogate to tumor response to treatment. MRI approaches using magnetic NPs have recently been validated for the examination of tumor vascularity in vivo 82. Therefore, Guimaraes et al examined a mouse model for RCC and followed tumor vascularity change using magnetic NPs after treatment with both mTOR inhibitor and TKI 83. The study observed marked reduction in tumor vascularity in both groups from baseline and control untreated groups of animals. The advantage to this potential monitoring technique would be measuring response to therapy, act as a surrogate biomarker of anti-angiogenic therapy. NP imaging targeting tumor vascularity could serve as a preclinical outcome measure of dose scheduling, and allow for pretreatment determination of potential therapeutic efficacy with those tumors demonstrating low vascular volume fraction, possibly precluding anti-angiogenic treatment strategies 82,83.
An unusual application of NPs for renal imaging has been reported in a case of splenosis 84. A patient, who formerly underwent splenectomy, was found to have a left renal mass on cross sectional imaging. Using ferumoxide, dextran-coated superparamagnetic iron oxides preferentially taken up by Kupffer cells of the RES, the kidney parenchyma was able to be distinguished from the ectopic autotransplanted splenic tissue. This is evident by loss of signal on T2-star imaging 84. The patient was spared from undergoing nephrectomy.
Metastatic treatment
Cytokine therapy with interleukin-2 or interferon-alpha have been standard of care for many years for treating metastatic RCC and have response rates of ~15% 85. 2-methoxyestradiol (2ME2) is a non-estrogenic derivative of estradiol with antiproliferative and antiangiogenic activity that downregulates hypoxia inducible factor alpha (HIF-1α) 86. The capsule formulation of 2ME2 showed minimal plasma levels and could not be evaluated for therapeutic potential 87. NPs of 2ME2 were created and have undergone investigation in a Phase I trial in patients with metastatic RCC who progressed on a TKI 88. Because of the response of several patients, a Phase II study was undertaken with 17 patients either receiving 2ME2 NPs alone or in combination with sunitinib. No observed objective response was demonstrated at the recommended dose and a significant number of patients required discontinued treatment due to toxicities 86.
Gene therapy is an active area of cancer research, offering the ability to target the base cause of cancer regulating genes. However, one challenge of gene therapy is delivery in vivo as DNA and RNA typically have short half-lives in circulation 89. The encapsulation of nucleic acids by PEG offers protection in vivo from enzymatic and immune mediated degredation and increases their size, preventing renal clearance 90. As metastatic RCC is not sensitive to radiotherapy or chemotherapy, immunomodulation and gene therapy have been targets of new research. Gene therapy has been investigated using polyethleneimine (PEI) as a delivery system in nanocomplexes through electrostatic interactions with DNA. However, the PEI is cytotoxic to the point of ineffectiveness 91. Xu et al evaluated 3 modifications of PEI in terms of cytotoxicity and transfection efficacy in an RCC mouse model 92. They found that all modified nanoparticles had lower cytotoxicity compared to PEI and furthermore one (a folic acid modified NP) had improved transfection efficiency in vitro and in vivo, reducing tumor volume by 30% compared to control groups 92.
In our approach, we have been developing PLGA nanoparticles as a sustained nonviral gene expression vector and demonstrated their efficacy with tumor suppressor p53 gene in different tumor models 93,94. It has been known that tumors with p53 mutation are associated with more aggressive disease, as well as increased resistance to chemotherapy and radiotherapy. Targeting gene- or drug-loaded nanoparticles (NPs) to tumors and ensuring their intratumoral retention after systemic administration remain key challenges to improving the efficacy of NP-based therapeutics. In this regard, we have been investigating a novel targeting approach that exploits changes in lipid metabolism and cell membrane biophysics that occur during malignancy. Recently, we have demonstrated that surface modified NPs which show greater biophysical interactions with the lipids of cancer cells than with the normal cell lipids show increased NP uptake by cancer cells in vitro, enhanced tumor accumulation in vivo, and improved efficacy of gene therapy 95. In another study, we are exploring biophysical interactions with the lipids of drug resistant cells in order to develop NPs that can overcome drug resistance 96,97. Our studies suggest a role of NP-lipid biophysical interactions in developing NP-based therapeutics. Thus, characterization of the biophysical interactions between NPs with different surface characteristics and lipid membranes of tumors may be a promising approach for screening and developing targeted delivery systems 98.
Another target of research involves vascular endothelium and limiting angiogenesis in tumors. Integin ανβ3, an internalization receptor for a number of viruses, has been shown to be expressed on angiogenic endothelium in malignant or diseased tissues 99. Murphy et al designed ανβ3-targeted NPs capable of delivering pharmacological agents to the ανβ3-expressing tumor vasculature 100. A metastatic RCC mouse model was used and doxorubicin was loaded into NPs. The results showed improvement in incidence of metastatic disease and total metastatic burden as compared to drug alone as well as yielding limited side effects 100.
Tetraiodothyroacetic acid (Tetrac), a thyroid hormone antagonist, has previously demonstrated antiproliferation effect on cells 101. Tetrac acts as an antagonist at the integrin receptor on the cell surface and suppresses angiogenesis along with its binding to thyroid hormone receptor within the cell. To determine the mechanism of tumor growth suppression of Tetrac, PLGA NPs encapsulating Tetrac were created with a size of 200 nm, prohibiting uptake into cells 102. In a mouse model of RCC, it was shown that both tetrac and tetrac NP have similar effects on inhibition of cancer cell proliferation and angiogenesis supporting the notion that tetrac acts on the integrin ανβ3 receptor on the plasma membrane 102. Thus, there may be an application for NP encapsulated Tetrac where the effects of thyroid hormone receptor would be avoided.
Local treatment
Localized RCC can be cured with surgical excision; however survival decreases dramatically with lymph node involvement. There exists conflicting evidence regarding the benefits of lymph node dissection (LND) at the time of radical nephrectomy for clinically localized RCC103–106. Potentially, the utility of LND would be realized if the correct patients were selected with more sophisticated pre-operative imaging identifying lymph node involvement. Current imaging with CT, MRI, and PET have limited capabilities for distinguishing metastatic lymph node involvement, specifically smaller sized nodes106. Lymphotrophic NP-enhanced MRI has been shown to be effective and accurate at distinguishing benign from malignant lymph nodes in prostate cancer107. In 9 patients with imaging suggesting localized RCC, lymphotrophic NP-enhanced MRI was performed with and without magnetic NPs called ferumoxtran-10 (Combidex, AMAG Pharmaceuticals, Inc.) followed by radical nephrectomy and LND 108. Although small in patient size and number of metastatic events, the study showed high sensitivity (100%) and specificity (95.7%) for distinguishing metastatic lymph nodes with magnetic NP-enhanced MRI.
Radiofrequency ablation (RFA) for treatment of small renal masses has become widely accepted due to its minimally invasive nature and ability to spare nephrons. However, tumors amenable to RFA are restricted by size and proximity to hilar structures or adjacent organs. During RFA, tumor is destroyed most completely at the center of the inner zone and from there circumferentially cell death dissipates. RFA efficiency would increase if the region of total cell death could be increased without expanding the total area of thermal injury. Tumor necrosis factor-α (TNF-α) targets tumor cells and vasculature, but has dose-limiting toxicities when administered systemically 109. Gold NPs loaded with TNF-α have been previously shown to accumulate within tumor interstitium with reduced systemic toxicity 110. From these studies, it was then shown that gold coated TNF-α NPs were able to sensitize the tumor so that the region of cell death when injected prior to RFA was increased by 23% 109.
Another minimally invasive approach to treating RCC involves magnetic thermoablation, or the application of energy on targeted tissue to achieve necrosis. Iron oxide NPs are exposed to an alternating electromagnetic field producing heat 111. Using these NPs as a ferrofluid, a rabbit model for RCC was created and the ferrofluid injected into the tumor under CT-guidance 112. The animal was exposed to an alternating electromagnetic field for 15 minutes and then the organs harvested for assessment. They found that percutaneous injection of the fluid was technically feasible, although there existed variability in how much tumor tissue showed histologic evidence of treatment in each animal as there remained areas free of necrosis 112. A more complete method of delivery of the ferrofluid to the tumor is needed, but this study does show the feasibility as well as the ability to monitor the effects of ferrofluid theromablation by CT scan.
Nephrotoxicity of Nanoparticles
Tissue distribution and toxicity of IV or GI administered biological NPs are of interest as the field of nanomedicine grows. Toxicity to the kidneys is often a limit of drug delivery, which has not been extensively investigated with NPs in particular. SiO2 NPs have been investigated in a number of biomedical systems. Wang et al studied the toxicity of SiO2 NPs in vitro with an embryonic kidney cell line and showed that cell viability decreased with an increase in dose and increase in time exposed113. Microscopically, cells exhibited features of apoptosis. NPs of 20 nm were more toxic than 50 nm NPs, likely owning to how the renal filtration handles particles of different sizes. Patra et al investigated toxicity of gold NPs on 3 different cell lines in vitro, one of which was baby hamster kidney114. This cell line showed no effect on cell morphology or cell viability after treatment with the gold NPs at various doses (versus substantial morphologic changes and decreased survival in a liver cell line). Toxicity of NPs used for enhanced imaging has been studied in vivo. Sadaf et al examined quantum dot NPs with silica coating administer intravenously to mice and found no increased in measure serum urea nitrogen or creatinine levels as well as no morphologic changes on histochemistry examination115. Sodium-oleate-coated USPIO have been studied in vivo in a rat model administered as a single dose at varying concentrations and followed for 1 month. There was no change in renal function or cellular morphology116. While these studies highlight some of the properties of NPs and how they may affect the kidney, there exist no in vivo human studies that particularly investigate the toxicology profiles of these biological NPs.
Summary
This review highlights areas of active clinical practice with NPs in the fields of nephrology and urology. NPs have been deployed in renal imaging techniques and delivery of iron therapy with Ferumoxytol for CKD or ESRD patients who lack appropriate erythropoietin production. There exist active areas of research with NPs in animal models. Hypertensive and atherosclerotic rodents have shown major improvements in disease state with brief treatment periods. NPs are able to sensitize renal tumors to minimal invasive techniques such as RFA for improved cell necrosis. Magnetic assisted hemodialysis with toxin scavenging NPs shows promise in vitro of improved solute clearance in shorter dialysis sessions, which would greatly improve the quality of life of these patients.
The strategies and advancements reported here are merely a sliver of the possibilities that NPs could contribute to advancing the treatment of kidney diseases. Challenges exist regarding translating the work from the bench to bedside, limiting clearance of the NPs by the kidney while targeting treatment to the appropriate tissue or area of disease, and demonstrating the biocompatibility of different nanostructures.
Acknowledgments
The work described here from authors’ laboratory is funded by Grant 1R01CA149359 (to V.L.) from the National Cancer Institute (NCI) of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Neuwelt EA, Hamilton BE, Varallyay CG, et al. Ultrasmall superparamagnetic iron oxides (USPIOs): a future alternative magnetic resonance (MR) contrast agent for patients at risk for nephrogenic systemic fibrosis (NSF)? Kidney Int. 2009 Mar;75(5):465–474. doi: 10.1038/ki.2008.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arsalani N, Fattahi H, Laurent S, Burtea C, Vander Elst L, Muller RN. Polyglycerol-grafted superparamagnetic iron oxide nanoparticles: highly efficient MRI contrast agent for liver and kidney imaging and potential scaffold for cellular and molecular imaging. Contrast Media Mol Imaging. 2012 Mar-Apr;7(2):185–194. doi: 10.1002/cmmi.479. [DOI] [PubMed] [Google Scholar]
- 3.Hultman KL, Raffo AJ, Grzenda AL, Harris PE, Brown TR, O’Brien S. Magnetic resonance imaging of major histocompatibility class II expression in the renal medulla using immunotargeted superparamagnetic iron oxide nanoparticles. ACS nano. 2008 Mar;2(3):477–484. doi: 10.1021/nn700400h. [DOI] [PubMed] [Google Scholar]
- 4.Bhardwaj V, Hariharan S, Bala I, et al. Pharmaceutical Aspects of Polymeric Nanoparticles for Oral Drug Delivery. J Biomed Nanotechnol. 2005 Sep;1(3):235–258. [Google Scholar]
- 5.Mu L, Feng SS. A novel controlled release formulation for the anticancer drug paclitaxel (Taxol): PLGA nanoparticles containing vitamin E TPGS. J Control Release. 2003 Jan 9;86(1):33–48. doi: 10.1016/s0168-3659(02)00320-6. [DOI] [PubMed] [Google Scholar]
- 6.des Rieux A, Fievez V, Garinot M, Schneider YJ, Preat V. Nanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approach. J Control Release. 2006 Nov;116(1):1–27. doi: 10.1016/j.jconrel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 7.Vila A, Sanchez A, Tobio M, Calvo P, Alonso MJ. Design of biodegradable particles for protein delivery. J Control Release. 2002 Jan 17;78(1–3):15–24. doi: 10.1016/s0168-3659(01)00486-2. [DOI] [PubMed] [Google Scholar]
- 8.Yoncheva K, Lizarraga E, Irache JM. Pegylated nanoparticles based on poly(methyl vinyl ether-co-maleic anhydride): preparation and evaluation of their bioadhesive properties. Eur J Pharm Sci. 2005 Apr;24(5):411–419. doi: 10.1016/j.ejps.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Zheng X, Lu J, Deng L, Xiong Y, Chen J. Preparation and characterization of magnetic cationic liposome in gene delivery. Int J Pharm. 2009 Jan 21;366(1–2):211–217. doi: 10.1016/j.ijpharm.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 10.Ruoslahti E. Peptides as targeting elements and tissue penetration devices for nanoparticles. Adv Mater. 2012 Jul 24;24(28):3747–3756. doi: 10.1002/adma.201200454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discov. 2003 May;2(5):347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 12.Choi CH, Zuckerman JE, Webster P, Davis ME. Targeting kidney mesangium by nanoparticles of defined size. Proc Natl Acad Sci U S A. 2011 Apr 19;108(16):6656–6661. doi: 10.1073/pnas.1103573108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choyke PL, Kobayashi H. Functional magnetic resonance imaging of the kidney using macromolecular contrast agents. Abdom Imaging. 2006 Mar-Apr;31(2):224–231. doi: 10.1007/s00261-005-0390-9. [DOI] [PubMed] [Google Scholar]
- 14.Weissleder R, Elizondo G, Wittenberg J, Lee AS, Josephson L, Brady TJ. Ultrasmall superparamagnetic iron oxide: an intravenous contrast agent for assessing lymph nodes with MR imaging. Radiology. 1990 May;175(2):494–498. doi: 10.1148/radiology.175.2.2326475. [DOI] [PubMed] [Google Scholar]
- 15.Jendelova P, Herynek V, Urdzikova L, et al. Magnetic resonance tracking of transplanted bone marrow and embryonic stem cells labeled by iron oxide nanoparticles in rat brain and spinal cord. J Neurosci Res. 2004 Apr 15;76(2):232–243. doi: 10.1002/jnr.20041. [DOI] [PubMed] [Google Scholar]
- 16.Jain TK, Morales MA, Sahoo SK, Leslie-Pelecky DL, Labhasetwar V. Iron oxide nanoparticles for sustained delivery of anticancer agents. Mol Pharmaceutics. 2005 May-Jun;2(3):194–205. doi: 10.1021/mp0500014. [DOI] [PubMed] [Google Scholar]
- 17.Li C, Li L, Keates AC. Targeting cancer gene therapy with magnetic nanoparticles. Oncotarget. 2012 Apr;3(4):365–370. doi: 10.18632/oncotarget.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar A, Jena PK, Behera S, Lockey RF, Mohapatra S, Mohapatra S. Multifunctional magnetic nanoparticles for targeted delivery. Nanomedicine. 2010 Feb;6(1):64–69. doi: 10.1016/j.nano.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ankola DD, Viswanad B, Bhardwaj V, Ramarao P, Kumar MN. Development of potent oral nanoparticulate formulation of coenzyme Q10 for treatment of hypertension: can the simple nutritional supplements be used as first line therapeutic agents for prophylaxis/therapy? Eur J Pharm Biopharm. 2007 Sep;67(2):361–369. doi: 10.1016/j.ejpb.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Aberg F, Appelkvist EL, Dallner G, Ernster L. Distribution and redox state of ubiquinones in rat and human tissues. Arch Biochem Biophys. 1992 Jun;295(2):230–234. doi: 10.1016/0003-9861(92)90511-t. [DOI] [PubMed] [Google Scholar]
- 21.Langsjoen H, Langsjoen P, Langsjoen P, Willis R, Folkers K. Usefulness of coenzyme Q10 in clinical cardiology: a long-term study. Mol Aspects Med. 1994;15( Suppl):s165–175. doi: 10.1016/0098-2997(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 22.Joo JS. Coenzyme Q-10 and Cardiovascular Health: To Take or Not to Take – That is the Question. Nutrition Bytes. 2005;10(2) [Google Scholar]
- 23.Digiesi V, Cantini F, Oradei A, et al. Coenzyme Q10 in essential hypertension. Mol Aspects Med. 1994;15( Suppl):s257–263. doi: 10.1016/0098-2997(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 24.McCarty MF. Coenzyme Q versus hypertension: does CoQ decrease endothelial superoxide generation? Med Hypotheses. 1999 Oct;53(4):300–304. doi: 10.1054/mehy.1997.0761. [DOI] [PubMed] [Google Scholar]
- 25.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res. 1996 Mar;78(3):415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- 26.Spector AA, Fang X, Snyder GD, Weintraub NL. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog Lipid Res. 2004 Jan;43(1):55–90. doi: 10.1016/s0163-7827(03)00049-3. [DOI] [PubMed] [Google Scholar]
- 27.Yu Z, Xu F, Huse LM, et al. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circ Res. 2000 Nov 24;87(11):992–998. doi: 10.1161/01.res.87.11.992. [DOI] [PubMed] [Google Scholar]
- 28.Ghosh S, Chiang PC, Wahlstrom JL, Fujiwara H, Selbo JG, Roberds SL. Oral delivery of 1,3-dicyclohexylurea nanosuspension enhances exposure and lowers blood pressure in hypertensive rats. Basic Clin Pharmacol Toxicol. 2008 May;102(5):453–458. doi: 10.1111/j.1742-7843.2008.00213.x. [DOI] [PubMed] [Google Scholar]
- 29.Mahfouz RA, El Tahlawi MA, Ateya AA, Elsaied A. Early detection of silent ischemia and diastolic dysfunction in asymptomatic young hypertensive patients. Echocardiography. 2011 May;28(5):564–569. doi: 10.1111/j.1540-8175.2011.01388.x. [DOI] [PubMed] [Google Scholar]
- 30.Norat T, Bowman R, Luben R, et al. Blood pressure and interactions between the angiotensin polymorphism AGT M235T and sodium intake: a cross-sectional population study. Am J Clin Nutr. 2008 Aug;88(2):392–397. doi: 10.1093/ajcn/88.2.392. [DOI] [PubMed] [Google Scholar]
- 31.Lu P, Yuan L, Wang Y, Du Q, Sheng J. Effect of GPE-AGT nanoparticle shRNA transfection system mediated RNAi on early atherosclerotic lesion. Int J Clin Exp Pathol. 2012;5(7):698–706. [PMC free article] [PubMed] [Google Scholar]
- 32.Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. J Am Med Assoc. 2005 Aug 17;294(7):813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 33.Waikar SS, Bonventre JV. Creatinine Kinetics and the Definition of Acute Kidney Injury. J Am Soc Nephrol. 2009 Mar;20(3):672–679. doi: 10.1681/ASN.2008070669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kobayashi H, Jo SK, Kawamoto S, et al. Polyamine dendrimer-based MRI contrast agents for functional kidney imaging to diagnose acute renal failure. J Magn Reson Imaging. 2004 Sep;20(3):512–518. doi: 10.1002/jmri.20147. [DOI] [PubMed] [Google Scholar]
- 35.Dear JW, Kobayashi H, Brechbiel MW, Star RA. Imaging acute renal failure with polyamine dendrimer-based MRI contrast agents. Nephron Clinical practice. 2006;103(2):c45–49. doi: 10.1159/000090608. [DOI] [PubMed] [Google Scholar]
- 36.Cattell V. Macrophages in acute glomerular inflammation. Kidney Int. 1994 Apr;45(4):945–952. doi: 10.1038/ki.1994.128. [DOI] [PubMed] [Google Scholar]
- 37.Grau V, Herbst B, Steiniger B. Dynamics of monocytes/macrophages and T lymphocytes in acutely rejecting rat renal allografts. Cell Tissue Res. 1998 Jan;291(1):117–126. doi: 10.1007/s004410050985. [DOI] [PubMed] [Google Scholar]
- 38.Schreiner GF, Harris KP, Purkerson ML, Klahr S. Immunological aspects of acute ureteral obstruction: immune cell infiltrate in the kidney. Kidney Int. 1988 Oct;34(4):487–493. doi: 10.1038/ki.1988.207. [DOI] [PubMed] [Google Scholar]
- 39.Shirai T, Kohara H, Tabata Y. Inflammation imaging by silica nanoparticles with antibodies orientedly immobilized. J Drug Target. 2012 Jul;20(6):535–543. doi: 10.3109/1061186X.2012.693500. [DOI] [PubMed] [Google Scholar]
- 40.Padanilam BJ. Cell death induced by acute renal injury: a perspective on the contributions of apoptosis and necrosis. Am J Physiol Renal Physiol. 2003 Apr;284(4):F608–627. doi: 10.1152/ajprenal.00284.2002. [DOI] [PubMed] [Google Scholar]
- 41.Bonventre JV. Mechanisms of ischemic acute renal failure. Kidney international. 1993 May;43(5):1160–1178. doi: 10.1038/ki.1993.163. [DOI] [PubMed] [Google Scholar]
- 42.Vetterlein F, Bludau J, Petho-Schramm A, Schmidt G. Reconstruction of blood flow distribution in the rat kidney during postischemic renal failure. Nephron. 1994;66(2):208–214. doi: 10.1159/000187802. [DOI] [PubMed] [Google Scholar]
- 43.Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol. 2001 Nov;281(5):F887–899. doi: 10.1152/ajprenal.2001.281.5.F887. [DOI] [PubMed] [Google Scholar]
- 44.Chien CT, Lee PH, Chen CF, Ma MC, Lai MK, Hsu SM. De novo demonstration and co-localization of free-radical production and apoptosis formation in rat kidney subjected to ischemia/reperfusion. J Am Soc Nephrol. 2001 May;12(5):973–982. doi: 10.1681/ASN.V125973. [DOI] [PubMed] [Google Scholar]
- 45.Tilney NL, Paz D, Ames J, Gasser M, Laskowski I, Hancock WW. Ischemia-reperfusion injury. Transplant Proc. 2001 Feb-Mar;33(1–2):843–844. doi: 10.1016/s0041-1345(00)02341-1. [DOI] [PubMed] [Google Scholar]
- 46.Mason J, Joeris B, Welsch J, Kriz W. Vascular congestion in ischemic renal failure: the role of cell swelling. Miner Electrolyte Metab. 1989;15(3):114–124. [PubMed] [Google Scholar]
- 47.Vasko KA, DeWall RA, Riley AM. Effect of allopurinol in renal ischemia. Surgery. 1972 May;71(5):787–790. [PubMed] [Google Scholar]
- 48.Paller MS, Hedlund BE. Role of iron in postischemic renal injury in the rat. Kidney Int. 1988 Oct;34(4):474–480. doi: 10.1038/ki.1988.205. [DOI] [PubMed] [Google Scholar]
- 49.Sheridan AM, Bonventre JV. Cell biology and molecular mechanisms of injury in ischemic acute renal failure. Curr Opin Nephrol Hypertens. 2000 Jul;9(4):427–434. doi: 10.1097/00041552-200007000-00015. [DOI] [PubMed] [Google Scholar]
- 50.Willinger CC, Schramek H, Pfaller K, Pfaller W. Tissue distribution of neutrophils in postischemic acute renal failure. Virchows Arch B Cell Pathol Incl Mol Pathol. 1992;62(4):237–243. doi: 10.1007/BF02899687. [DOI] [PubMed] [Google Scholar]
- 51.Kim J, Seok YM, Jung KJ, Park KM. Reactive oxygen species/oxidative stress contributes to progression of kidney fibrosis following transient ischemic injury in mice. Am J Physiol Renal Physiol. 2009 Aug;297(2):F461–470. doi: 10.1152/ajprenal.90735.2008. [DOI] [PubMed] [Google Scholar]
- 52.Domanski L, Dolegowska B, Safranow K, et al. Activity of CuZn-superoxide dismutase, catalase and glutathione peroxidase in erythrocytes in kidney allografts during reperfusion in patients with and without delayed graft function. Clin Transplant. 2006 Jan-Feb;20(1):67–71. doi: 10.1111/j.1399-0012.2005.00442.x. [DOI] [PubMed] [Google Scholar]
- 53.Hood E, Simone E, Wattamwar P, Dziubla T, Muzykantov V. Nanocarriers for vascular delivery of antioxidants. Nanomedicine. 2011 Sep;6(7):1257–1272. doi: 10.2217/nnm.11.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen YP, Chen CT, Hung Y, et al. A new strategy for intracellular delivery of enzyme using mesoporous silica nanoparticles: superoxide dismutase. J Am Chem Soc. 2013 Jan 30;135(4):1516–1523. doi: 10.1021/ja3105208. [DOI] [PubMed] [Google Scholar]
- 55.Reddy MK, Labhasetwar V. Nanoparticle-mediated delivery of superoxide dismutase to the brain: an effective strategy to reduce ischemia-reperfusion injury. FASEB J. 2009 May;23(5):1384–1395. doi: 10.1096/fj.08-116947. [DOI] [PubMed] [Google Scholar]
- 56.Yun X, Maximov VD, Yu J, Zhu H, Vertegel AA, Kindy MS. Nanoparticles for targeted delivery of antioxidant enzymes to the brain after cerebral ischemia and reperfusion injury. J Cereb Blood Flow Metab. 2013 Apr;33(4):583–592. doi: 10.1038/jcbfm.2012.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ye Q, Yang D, Williams M, et al. In vivo detection of acute rat renal allograft rejection by MRI with USPIO particles. Kidney Int. 2002 Mar;61(3):1124–1135. doi: 10.1046/j.1523-1755.2002.00195.x. [DOI] [PubMed] [Google Scholar]
- 58.Wu YL, Ye Q, Foley LM, et al. In situ labeling of immune cells with iron oxide particles: an approach to detect organ rejection by cellular MRI. Proc Natl Acad Sci U S A. 2006 Feb 7;103(6):1852–1857. doi: 10.1073/pnas.0507198103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hauger O, Delalande C, Trillaud H, et al. MR imaging of intrarenal macrophage infiltration in an experimental model of nephrotic syndrome. Magn Reson Med. 1999 Jan;41(1):156–162. doi: 10.1002/(sici)1522-2594(199901)41:1<156::aid-mrm22>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 60.Laissy JP, Idee JM, Loshkajian A, et al. Reversibility of experimental acute renal failure in rats: assessment with USPIO-enhanced MR imaging. J Magn Reson Imaging. 2000 Aug;12(2):278–288. doi: 10.1002/1522-2586(200008)12:2<278::aid-jmri10>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Y, Dodd SJ, Hendrich KS, Williams M, Ho C. Magnetic resonance imaging detection of rat renal transplant rejection by monitoring macrophage infiltration. Kidney Int. 2000 Sep;58(3):1300–1310. doi: 10.1046/j.1523-1755.2000.00286.x. [DOI] [PubMed] [Google Scholar]
- 62.Italia JL, Bhardwaj V, Kumar MN. Disease, destination, dose and delivery aspects of ciclosporin: the state of the art. Drug Discov Today. 2006 Sep;11(17–18):846–854. doi: 10.1016/j.drudis.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 63.Dai J, Nagai T, Wang X, Zhang T, Meng M, Zhang Q. pH-sensitive nanoparticles for improving the oral bioavailability of cyclosporine A. Int J Pharm. 2004 Aug 6;280(1–2):229–240. doi: 10.1016/j.ijpharm.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 64.Lai J, Lu Y, Yin Z, Hu F, Wu W. Pharmacokinetics and enhanced oral bioavailability in beagle dogs of cyclosporine A encapsulated in glyceryl monooleate/poloxamer 407 cubic nanoparticles. Int J Nanomedicine. 2010;5:13–23. [PMC free article] [PubMed] [Google Scholar]
- 65.Devarajan P. Review: neutrophil gelatinase-associated lipocalin: a troponin-like biomarker for human acute kidney injury. Nephrology. 2010 Jun;15(4):419–428. doi: 10.1111/j.1440-1797.2010.01317.x. [DOI] [PubMed] [Google Scholar]
- 66.Simenhoff ML, Burke JF, Saukkonen JJ, Ordinario AT, Doty R. Biochemical profile or uremic breath. N Engl J Med. 1977 Jul 21;297(3):132–135. doi: 10.1056/NEJM197707212970303. [DOI] [PubMed] [Google Scholar]
- 67.Haick H, Hakim M, Patrascu M, et al. Sniffing chronic renal failure in rat model by an array of random networks of single-walled carbon nanotubes. ACS nano. 2009 May 26;3(5):1258–1266. doi: 10.1021/nn9001775. [DOI] [PubMed] [Google Scholar]
- 68.Marom O, Nakhoul F, Tisch U, Shiban A, Abassi Z, Haick H. Gold nanoparticle sensors for detecting chronic kidney disease and disease progression. Nanomedicine. 2012 May;7(5):639–650. doi: 10.2217/nnm.11.135. [DOI] [PubMed] [Google Scholar]
- 69.Stamopoulos D, Benaki D, Bouziotis P, Zirogiannis PN. In vitro utilization of ferromagnetic nanoparticles in hemodialysis therapy. Nanotechnology. 2007 Dec 12;18(49):495102. doi: 10.1088/0957-4484/18/49/495102. [DOI] [PubMed] [Google Scholar]
- 70.Schwenk MH. Ferumoxytol: a new intravenous iron preparation for the treatment of iron deficiency anemia in patients with chronic kidney disease. Pharmacotherapy. 2010 Jan;30(1):70–79. doi: 10.1592/phco.30.1.70. [DOI] [PubMed] [Google Scholar]
- 71.Swinkels DW, Wetzels JF. Hepcidin: a new tool in the management of anaemia in patients with chronic kidney disease? Nephrol Dial Transplant. 2008 Aug;23(8):2450–2453. doi: 10.1093/ndt/gfn267. [DOI] [PubMed] [Google Scholar]
- 72.Balakrishnan VS, Rao M, Kausz AT, et al. Physicochemical properties of ferumoxytol, a new intravenous iron preparation. Eur J Clin Invest. 2009 Jun;39(6):489–496. doi: 10.1111/j.1365-2362.2009.02130.x. [DOI] [PubMed] [Google Scholar]
- 73.Spinowitz BS, Kausz AT, Baptista J, et al. Ferumoxytol for treating iron deficiency anemia in CKD. J Am Soc Nephrol. 2008 Aug;19(8):1599–1605. doi: 10.1681/ASN.2007101156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Provenzano R, Schiller B, Rao M, Coyne D, Brenner L, Pereira BJ. Ferumoxytol as an intravenous iron replacement therapy in hemodialysis patients. Clin J Am Soc Nephrol. 2009 Feb;4(2):386–393. doi: 10.2215/CJN.02840608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Singh A, Patel T, Hertel J, Bernardo M, Kausz A, Brenner L. Safety of ferumoxytol in patients with anemia and CKD. Am J Kidney Dis. 2008 Nov;52(5):907–915. doi: 10.1053/j.ajkd.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 76.Mahmood U. Science to practice: can a targeted nanoparticle be used to image autoimmune nephritis? Radiology. 2010 May;255(2):309–310. doi: 10.1148/radiol.100186. [DOI] [PubMed] [Google Scholar]
- 77.Thurman JM, Rohrer B. Noninvasive detection of complement activation through radiologic imaging. Adv Exp Med Biol. 2013;735:271–282. doi: 10.1007/978-1-4614-4118-2_19. [DOI] [PubMed] [Google Scholar]
- 78.Serkova NJ, Renner B, Larsen BA, et al. Renal inflammation: targeted iron oxide nanoparticles for molecular MR imaging in mice. Radiology. 2010 May;255(2):517–526. doi: 10.1148/radiol.09091134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ix JH, Mercado N, Shlipak MG, et al. Association of chronic kidney disease with clinical outcomes after coronary revascularization: the Arterial Revascularization Therapies Study (ARTS) Am Heart J. 2005 Mar;149(3):512–519. doi: 10.1016/j.ahj.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 80.Goodwill PW, Saritas EU, Croft LR, et al. X-space MPI: magnetic nanoparticles for safe medical imaging. Adv Mater. 2012 Jul 24;24(28):3870–3877. doi: 10.1002/adma.201200221. [DOI] [PubMed] [Google Scholar]
- 81.Lu M, Cohen MH, Rieves D, Pazdur R. FDA report: Ferumoxytol for intravenous iron therapy in adult patients with chronic kidney disease. Am J Hematol. 2010 May;85(5):315–319. doi: 10.1002/ajh.21656. [DOI] [PubMed] [Google Scholar]
- 82.Bremer C, Mustafa M, Bogdanov A, Jr, Ntziachristos V, Petrovsky A, Weissleder R. Steady-state blood volume measurements in experimental tumors with different angiogenic burdens a study in mice. Radiology. 2003 Jan;226(1):214–220. doi: 10.1148/radiol.2261012140. [DOI] [PubMed] [Google Scholar]
- 83.Guimaraes AR, Ross R, Figuereido JL, Waterman P, Weissleder R. MRI with magnetic nanoparticles monitors downstream anti-angiogenic effects of mTOR inhibition. Mol Imaging Biol. 2011 Apr;13(2):314–320. doi: 10.1007/s11307-010-0357-2. [DOI] [PubMed] [Google Scholar]
- 84.Berman AJ, Zahalsky MP, Okon SA, Wagner JR. Distinguishing splenosis from renal masses using ferumoxide-enhanced magnetic resonance imaging. Urology. 2003 Oct;62(4):748. doi: 10.1016/s0090-4295(03)00509-0. [DOI] [PubMed] [Google Scholar]
- 85.Pyrhonen S, Salminen E, Ruutu M, et al. Prospective randomized trial of interferon alfa-2a plus vinblastine versus vinblastine alone in patients with advanced renal cell cancer. J Clin Oncol. 1999 Sep;17(9):2859–2867. doi: 10.1200/JCO.1999.17.9.2859. [DOI] [PubMed] [Google Scholar]
- 86.Bruce JY, Eickhoff J, Pili R, et al. A phase II study of 2-methoxyestradiol nanocrystal colloidal dispersion alone and in combination with sunitinib malate in patients with metastatic renal cell carcinoma progressing on sunitinib malate. Invest New Drugs. 2012 Apr;30(2):794–802. doi: 10.1007/s10637-010-9618-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dahut WL, Lakhani NJ, Gulley JL, et al. Phase I clinical trial of oral 2-methoxyestradiol, an antiangiogenic and apoptotic agent, in patients with solid tumors. Cancer Biol Ther. 2006 Jan;5(1):22–27. doi: 10.4161/cbt.5.1.2349. [DOI] [PubMed] [Google Scholar]
- 88.Tevaarwerk AJ, Holen KD, Alberti DB, et al. Phase I trial of 2-methoxyestradiol NanoCrystal dispersion in advanced solid malignancies. Clin Cancer Res. 2009 Feb 15;15(4):1460–1465. doi: 10.1158/1078-0432.CCR-08-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kole R, Krainer AR, Altman S. RNA therapeutics: beyond RNA interference and antisense oligonucleotides. Nat Rev Drug Discov. 2012 Feb;11(2):125–140. doi: 10.1038/nrd3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ogris M, Brunner S, Schuller S, Kircheis R, Wagner E. PEGylated DNA/transferrin-PEI complexes: reduced interaction with blood components, extended circulation in blood and potential for systemic gene delivery. Gene Ther. 1999 Apr;6(4):595–605. doi: 10.1038/sj.gt.3300900. [DOI] [PubMed] [Google Scholar]
- 91.Boussif O, Lezoualc’h F, Zanta MA, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci U S A. 1995 Aug 1;92(16):7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu Z, Shen G, Xia X, et al. Comparisons of three polyethyleneimine-derived nanoparticles as a gene therapy delivery system for renal cell carcinoma. J Transl Med. 2011;9(1):46. doi: 10.1186/1479-5876-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Prabha S, Sharma B, Labhasetwar V. Inhibition of tumor angiogenesis and growth by nanoparticle-mediated p53 gene therapy in mice. Cancer Gene Ther. 2012 Aug;19(8):530–537. doi: 10.1038/cgt.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sharma B, Ma W, Adjei IM, Panyam J, Dimitrijevic S, Labhasetwar V. Nanoparticle-mediated p53 gene therapy for tumor inhibition. Drug Deliv Transl Res. 2011 Feb;1(1):43–52. doi: 10.1007/s13346-010-0008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sharma B, Peetla C, Adjei IM, Labhasetwar V. Selective biophysical interactions of surface modified nanoparticles with cancer cell lipids improve tumor targeting and gene therapy. Cancer Lett. 2013 Mar 21; doi: 10.1016/j.canlet.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Peetla C, Bhave R, Vijayaraghavalu S, Stine A, Kooijman E, Labhasetwar V. Drug resistance in breast cancer cells: biophysical characterization of and doxorubicin interactions with membrane lipids. Mol Pharm. 2010 Dec 6;7(6):2334–2348. doi: 10.1021/mp100308n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vijayaraghavalu S, Peetla C, Lu S, Labhasetwar V. Epigenetic modulation of the biophysical properties of drug-resistant cell lipids to restore drug transport and endocytic functions. Mol Pharm. 2012 Sep 4;9(9):2730–2742. doi: 10.1021/mp300281t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Peetla C, Stine A, Labhasetwar V. Biophysical interactions with model lipid membranes: applications in drug discovery and drug delivery. Mol Pharm. 2009 Sep-Oct;6(5):1264–1276. doi: 10.1021/mp9000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brooks PC, Montgomery AM, Rosenfeld M, et al. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994 Dec 30;79(7):1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 100.Murphy EA, Majeti BK, Barnes LA, et al. Nanoparticle-mediated drug delivery to tumor vasculature suppresses metastasis. Proc Natl Acad Sci U S A. 2008 Jul 8;105(27):9343–9348. doi: 10.1073/pnas.0803728105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mousa SA, Bergh JJ, Dier E, et al. Tetraiodothyroacetic acid, a small molecule integrin ligand, blocks angiogenesis induced by vascular endothelial growth factor and basic fibroblast growth factor. Angiogenesis. 2008;11(2):183–190. doi: 10.1007/s10456-007-9088-7. [DOI] [PubMed] [Google Scholar]
- 102.Yalcin M, Bharali DJ, Lansing L, et al. Tetraidothyroacetic acid (tetrac) and tetrac nanoparticles inhibit growth of human renal cell carcinoma xenografts. Anticancer Res. 2009 Oct;29(10):3825–3831. [PubMed] [Google Scholar]
- 103.Giberti C, Oneto F, Martorana G, Rovida S, Carmignani G. Radical nephrectomy for renal cell carcinoma: long-term results and prognostic factors on a series of 328 cases. Eur Urol. 1997;31(1):40–48. doi: 10.1159/000474416. [DOI] [PubMed] [Google Scholar]
- 104.Giuliani L, Martorana G, Giberti C, Pescatore D, Magnani G. Results of radical nephrectomy with extensive lymphadenectomy for renal cell carcinoma. J Urol. 1983 Oct;130(4):664–668. doi: 10.1016/s0022-5347(17)51391-9. [DOI] [PubMed] [Google Scholar]
- 105.Blom JH, van Poppel H, Marechal JM, et al. Radical nephrectomy with and without lymph-node dissection: final results of European Organization for Research and Treatment of Cancer (EORTC) randomized phase 3 trial 30881. Eur Urol. 2009 Jan;55(1):28–34. doi: 10.1016/j.eururo.2008.09.052. [DOI] [PubMed] [Google Scholar]
- 106.Freedland SJ, Dekernion JB. Role of lymphadenectomy for patients undergoing radical nephrectomy for renal cell carcinoma. Rev Urol. 2003 Summer;5(3):191–195. [PMC free article] [PubMed] [Google Scholar]
- 107.Harisinghani MG, Weissleder R. Sensitive, noninvasive detection of lymph node metastases. PLoS medicine. 2004 Dec;1(3):e66. doi: 10.1371/journal.pmed.0010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guimaraes AR, Tabatabei S, Dahl D, McDougal WS, Weissleder R, Harisinghani MG. Pilot study evaluating use of lymphotrophic nanoparticle-enhanced magnetic resonance imaging for assessing lymph nodes in renal cell cancer. Urology. 2008 Apr;71(4):708–712. doi: 10.1016/j.urology.2007.11.096. [DOI] [PubMed] [Google Scholar]
- 109.Pedro RN, Thekke-Adiyat T, Goel R, et al. Use of tumor necrosis factor-alpha-coated gold nanoparticles to enhance radiofrequency ablation in a translational model of renal tumors. Urology. 2010 Aug;76(2):494–498. doi: 10.1016/j.urology.2010.01.085. [DOI] [PubMed] [Google Scholar]
- 110.Goel R, Shah N, Visaria R, Paciotti GF, Bischof JC. Biodistribution of TNF-alpha-coated gold nanoparticles in an in vivo model system. Nanomedicine. 2009 Jun;4(4):401–410. doi: 10.2217/nnm.09.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gneveckow U, Jordan A, Scholz R, et al. Description and characterization of the novel hyperthermia- and thermoablation-system MFH 300F for clinical magnetic fluid hyperthermia. Med Phys. 2004 Jun;31(6):1444–1451. doi: 10.1118/1.1748629. [DOI] [PubMed] [Google Scholar]
- 112.Bruners P, Braunschweig T, Hodenius M, et al. Thermoablation of malignant kidney tumors using magnetic nanoparticles: an in vivo feasibility study in a rabbit model. Cardiovasc Intervent Radiol. 2010 Feb;33(1):127–134. doi: 10.1007/s00270-009-9583-x. [DOI] [PubMed] [Google Scholar]
- 113.Wang F, Gao F, Lan M, Yuan H, Huang Y, Liu J. Oxidative stress contributes to silica nanoparticle-induced cytotoxicity in human embryonic kidney cells. Toxicol In Vitro. 2009 Aug;23(5):808–815. doi: 10.1016/j.tiv.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 114.Patra HK, Banerjee S, Chaudhuri U, Lahiri P, Dasgupta AK. Cell selective response to gold nanoparticles. Nanomedicine. 2007 Jun;3(2):111–119. doi: 10.1016/j.nano.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 115.Sadaf A, Zeshan B, Wang Z, et al. Toxicity evaluation of hydrophilic CdTe quantum dots and CdTe@SiO2 nanoparticles in mice. J Nanosci Nanotechnol. 2012 Nov;12(11):8287–8292. doi: 10.1166/jnn.2012.6667. [DOI] [PubMed] [Google Scholar]
- 116.Sebekova K, Dusinska M, Simon Klenovics K, et al. Comprehensive assessment of nephrotoxicity of intravenously administered sodium-oleate-coated ultra-small superparamagnetic iron oxide (USPIO) and titanium dioxide (TiO(2)) nanoparticles in rats. Nanotoxicology. 2013 Jan 21; doi: 10.3109/17435390.2012.763147. [DOI] [PubMed] [Google Scholar]