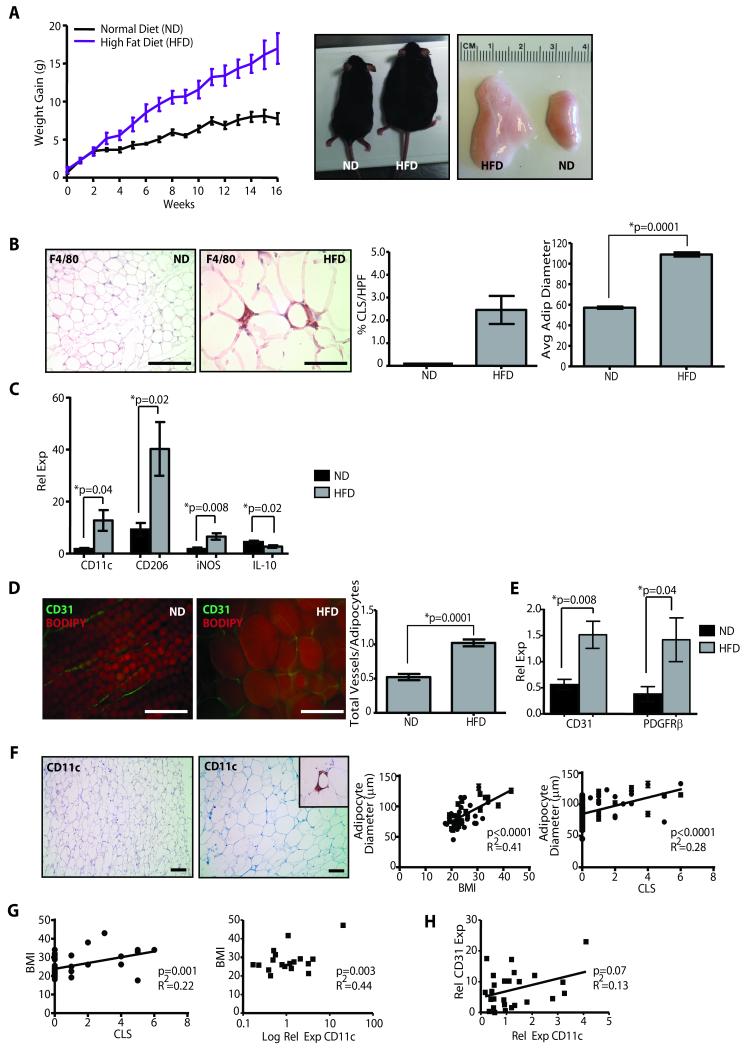
Figure 1. Mammary stroma recruits inflammatory macrophages and demonstrates increased vascularity under conditions of obesity.
(A) Female C57Bl/6 mice fed a high fat diet (HFD) gained significantly more weight, resulting in increased body weight (p=0.0006, n=10 mice/group) and mammary fat pad depot size (p=0.002), compared to those fed a normal chow diet (ND). (B) In obese mice, mammary fat depots demonstrated F4/80+ crown-like structures (CLS), which were not detectable in depots of control mice, as well as significantly increased adipocyte diameters. (C) Transcripts for M1-like (CD11c, iNOS) and M2-like (CD206) macrophage markers were elevated in mammary glands from HFD mice (qPCR, n=6 mice/group). (D) Mammary glands from obese mice demonstrated a significantly increased vessel to adipocyte ratio (n=5 mice/group). (E) Transcripts for CD31 and PDGFRβ were significantly elevated in glands from HFD mice (qPCR, n=6/group). (F) In breast tissue isolated from reduction mammoplasty surgeries, increasing adipocyte diameter was correlated with increased body mass index (BMI; n=46) as well as the formation of CD11c+ CLS (n=76). (G) Increased BMI was correlated with the formation of CD11c+ CLS (n=46), as well as increased relative expression of CD11c transcripts from reduction mammoplasty tissues (n=18). (H) Elevated CD11c expression was associated with increased CD31 expression (n=28). qPCR was performed on RNA isolated from reduction mammoplasty tissue. Original magnification (B, D) 200x, (F) 100x; bar=100μm.