Abstract
During the last three decades, 4-hydroxy-2-nonenal (HNE), a major α,β-unsaturated aldehyde product of n-6 fatty acid oxidation, has been shown to be involved in a great number of pathologies such as metabolic diseases, neurodegenerative diseases and cancers. These multiple pathologies can be explained by the fact that HNE is a potent modulator of numerous cell processes such as oxidative stress signaling, cell proliferation, transformation or cell death. The main objective of this review is to focus on the different aspects of HNE-induced cell death, with a particular emphasis on apoptosis. HNE is a special apoptotic inducer because of its abilities to form protein adducts and to propagate oxidative stress. It can stimulate intrinsic and extrinsic apoptotic pathways and interact with typical actors such as tumor protein 53, JNK, Fas or mitochondrial regulators. At the same time, due to its oxidant status, it can also induce some cellular defense mechanisms against oxidative stress, thus being involved in its own detoxification. These processes in turn limit the apoptotic potential of HNE. These dualities can imbalance cell fate, either toward cell death or toward survival, depending on the cell type, the metabolic state and the ability to detoxify.
Keywords: lipoperoxidation, apoptosis, detoxification, protein adduct, oxidative stress
Bullet Points
4-hydroxy-2-nonenal (HNE), a secondary product of lipoperoxidation, can form protein adducts and modifies cell signaling.
Because of its chemical reactivity, HNE can exert pleiotropic effects particularly in cell death.
HNE is accumulated in numerous oxidative stress-related diseases, such as neurodegenerative diseases (NDD), cardiovascular diseases, metabolic syndrome and cancer.
The regulation of cell death by HNE can impact the development of diseases associated with oxidative damage.
Open Questions
What are the mechanisms of cell death induced by HNE?
What is the relative importance of the canonical apoptotic pathways compared with atypical cell deaths in HNE-exposed cells?
Upon HNE exposure, what are the processes to avoid death?
How do the detoxification pathways modulate the cell fate upon HNE exposure?
4-Hydroxynonenal: a Highly Reactive Product of Lipoperoxidation
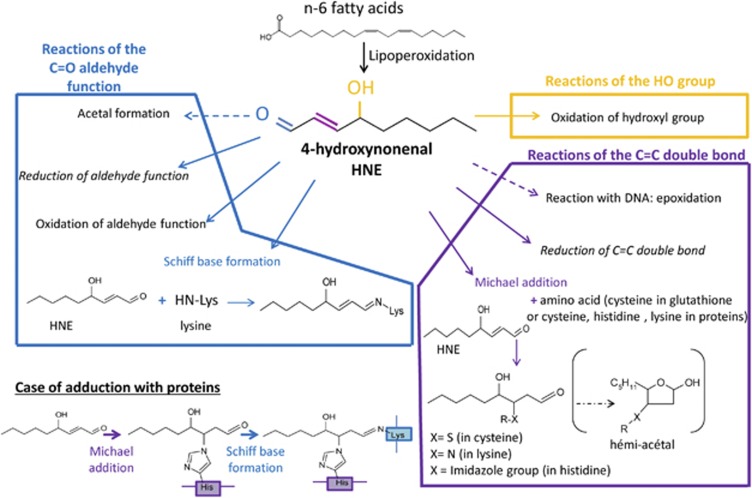
Non-enzymatic lipoperoxidation (LPO) is an autocatalytic process, initiated by the attack of free radicals on membrane polyunsaturated fatty acids (PUFAs). The α,β-unsaturated aldehyde 4-hydroxynonenal (HNE) is a major end product that is derived from the oxidation of n-6 PUFAs such as linoleic, γ-linolenic or arachidonic acids. HNE belongs to the advanced lipid peroxidation end products (ALEs). Despite its relative stability compared with free radicals, the chemical structure of HNE possesses three reactive functions: a C2=C3 double bond, a C1=O carbonyl group and a hydroxyl group on C4 (Figure 1). These reactive functions make this electrophilic molecule highly reactive toward nucleophilic thiol and amino groups. This reactivity relies upon both the Michael addition of thiol or amino compounds on the C3 of the C2=C3 double bond and the formation of Schiff bases between the C1 carbonyl group and primary amines. The kinetics of the Schiff base formation are inherently slow and reversible, making Michael-adducts predominant. HNE can then react with a large number of macromolecules such as proteins, principally those containing histidine, cysteine and lysine residues; lipids, which contains an amino group; and with nucleic acids, mostly with the guanosine moiety of DNA.1 These interactions with proteins, called HNE-protein adduction, modify their activity (for a review, see Schaur et al.1). Because of its double reactivity (Michael addition and Schiff bases), HNE can contribute to protein cross-linking and induce a carbonyl stress.
Figure 1.
Reactivity of HNE. Here are represented the potential reactions of HNE on the hydroxyl, carbonyl and double bond groups. HNE is biotransformed, but it can also react with proteins according to the Michael addition and the Schiff base formation and DNA by epoxidation
Under physiological conditions, cells have to cope with HNE stemming from different sources. The most common source is the endogenous one, coming from the reactive oxygen species (ROS) produced by the mitochondrial electron transport chain, which triggers lipid oxidation. When the cells are exposed to xenobiotics, the cytochrome P450 biotransformation activities can also generate ROS that are able to induce LPO. Thus, HNE generation has been associated with drugs or exposure to environmental contaminants, such as ethanol.2 Inflammation-related ROS are also a prominent source of HNE, and HNE-protein adducts are biomarkers in inflammatory diseases.3 Exogenous HNE is another way for the cells to be exposed. It can be produced by peroxidation of plasma low-density lipoproteins (LDL)4 or generated during food processing: the heme iron present in red meat can oxidize dietary polyunsaturated lipids.5 Intestinal cells are also major targets of exogenous HNE because they are at the interface with the lumen and can be directly exposed to high concentrations of HNE. Moreover, HNE can be produced by macrophages infected by gut microbiota and such generated HNE can be toxic for colon cells.6 As HNE is a highly diffusible molecule, it can spread beyond its initial production site. Based upon its diffusion capacity, it has been suggested that HNE can act as a paracrine signal molecule.7
Because of the basal level of ROS inherent to life under aerobic conditions, there should be a basal level of HNE in the cell. The HNE concentration in human blood and serum was estimated to be about 0.05–0.15 μM,8, 9 but in pathological situations and close to the core of LPO sites, its concentration can be greatly increased (more than 100 μM).10 Thus, high levels of HNE have been detected in a large number of diseases.11, 12 Nevertheless, even if HNE can be detected by several methods, acquiring reliable values of HNE in vivo is considerably compromised by its rapid metabolism, its efflux and its steady-state concentration in specific tissues.13 All cells are not equal regarding HNE detoxification. As an example, colonocytes metabolize 100% of 40 μM HNE in 90 min,14 whereas hepatocytes metabolize 95% of 100 μM HNE in 3 min.15 Therefore, the concentration of HNE and the duration of exposure can be modulated by the rate of detoxification in target organs.
Depending on its concentration and its targets, HNE can contribute to many biological functions: for example, stimulating or inhibiting enzymatic functions, such as kinases (PKC (protein kinase C).16 HNE interaction with key amino acids of catalytic sites could explain the inhibition of these enzymes.16 Although HNE-dependent protein modulation relies mainly on post-translational modifications, the regulation of gene expression by HNE has also been described notably by modulation of NF-κB (nuclear factor-kappa B) and AP-1 (activator protein 1) transcription factors, which are related to stress responses. It is particularly involved in the activation of the transcription factor Nrf2 (nuclear factor (erythroid-derived 2)-like 2), which transactivates the antioxidant responsive element (ARE). By doing so, it stimulates the cellular antioxidant defenses and the regulation of the oxidative stress, via the upregulation of the expression of various genes such as for heme oxygenase 1 (HO-1), aldehyde dehydrogenases (ALDH), glutathione S-transferase (GST), multidrug-resistant proteins (MRPs), aldose reductase (aldo-keto reductase, AKR), NADPH dehydrogenase quinone 1 (NQO1) or glutamate–cysteine ligase (GCL).17 Some of these genes were shown to be involved in HNE biotransformation.18 The physiological levels of HNE also have to be taken into account, as the depletion of HNE can affect gene expression such as Fas, Tp53, p21, c-myc or connexin 43.19
By its multiple impacts on protein regulation through transcriptional and post-translational modifications, HNE has a role in the maintenance of cellular homeostasis, during normal aerobic metabolism (low levels of ROS). However, higher and longer oxidative stress can lead to HNE accumulation thereby compromising cellular functions, as is the case in some pathological disorders.20
HNE and Diseases Associated with Oxidative Damage: Links to Cell Death
High levels of HNE have been associated with diseases involving redox imbalance: NDD,21 macular degeneration,22 cardiovascular diseases, atherosclerosis,23 metabolic syndrome24 and cancers.25 In these diseases, HNE is not only a simple marker of oxidative stress but also a causative agent. The modalities of HNE involvement in these oxidative stress-related diseases are detailed below and summarized in the Table 1.
Table 1. Involvement of HNE in diseases related to oxidative stress.
| Pathologies | In vivo and in vitro data | References |
|---|---|---|
| Alzheimer's disease | HNE-modified proteins ↗, proteasome activity ↘, inflammation ↗, neurodegeneration ↗ | 28, 29 |
| HNE-synaptosomal proteins conjugation, glucose transport ↘, mitochondrial ROS ↗, synaptic degeneration ↗ | 30 | |
| HNE-induced ion homeostasis disturbance, Na+/K+ ATPase activity ↘, free Ca2+ ↗, cell degeneration ↗ | 31 | |
| Parkinson's disease | HNE-modified proteins ↗, proteasome activity ↘, free radical generation ↗, oxidative stress ↗, dopaminergic cell death↗ | 32 |
| HNE-modified proteins ↗, proteasome activity ↘, free radical generation↗ | 33 | |
| Dopamine uptake ↘, Na+/K+ ATPase activity↘ | 35 | |
| Cancer | HNE-guanosine adducts, G C to T.A mutations on p53 ↗, DNA repair mechanisms ↘ | 60, 61, 62 |
| Low levels of HNE in tumor tissues compared with healthy tissues, ↘TGFβ1 | 46, 47, 4849 | |
| High levels of HNE in cancer tissues | 50, 51, 52, 53, 54, 55 | |
| Luminal HNE triggers the positive selection of preneoplastic cells in colorectal cancer | 14, 59 | |
| Atherosclerosis | HNE-induced oxidative stress, IL-8 ↘, ICAM-1 ↘, cytotoxicity↗, endothelial barrier abilities ↘, apoptosis ↗ | 40 |
| HNE-induced class A scavenger receptor synthesis, macrophage foam cells formation ↗, lipid cores formation ↗ | 37, 38 | |
| Liver diseases | HNE-induced JNK pathway, hepatocytes cell death ↗ (NAFLD) | 43 |
| HNE modification of ‘self'-proteins, autoimmune reactions ↗(ALD) | 2, 44 |
HNE, 4-hydroxy-2-nonenal; JNK, c-Jun N-terminal kinase; NAFLD, non-alcoholic fatty liver disease; ROS, reactive oxygen species; TGFβ1, transforming growth factor β
Special character for ‘arrow going up': increase; special character for ‘arrow going down': decrease.
Neurodegenerative diseases
In Alzheimer's disease, where increased oxidative damage in neuronal cell bodies is one of the earliest changes, HNE-adducts to neurofilaments have been found.26 It is the amyloid β-peptide that would induce oxidative stress and the consequent lipoperoxidation. High levels of HNE have been detected in amyloid β plaques and in the cerebrospinal fluid in Alzheimer's patients.27 The accumulation of HNE-modified amyloid β-peptides has been shown to inhibit the proteasome,28 and the resulting accumulation of ubiquitinated modified proteins leads to a pro-inflammatory response (cyclo-oxygenase 2, prostaglandins). This contributes to neurodegeneration.29 Moreover, by modifying membranes, HNE could impair Na+/Ca2+ pumps and glucose and glutamate transporters, leading to ionic and energetic disturbances and neuronal cell death.30, 31
In Parkinson's disease, oxidative stress is known to contribute to mitochondrial dysfunction and degeneration of dopaminergic cells.21 HNE can form adducts with proteins involved in the proteasome system, leading to its failure and neuronal cell death.32 HNE has been shown to be present in the mitochondria and Lewy bodies. In vitro experiments have revealed that the incubation of dopaminergic neurons with HNE leads to a decrease in dopamine uptake and a loss of Na+/K+ pump activity.33 Lipoperoxidation end product accumulation is not a simple tracer of oxidative stress but it can have a major role in the pathogenesis of Parkinson disease. HNE accumulation could be due to the impairment of the aldehyde detoxification system, among which are ALDH 1 and 2, both isoforms expressed in substantia nigra dopamine neurons.34 Null mice for ALDH1 and ADLH2 have high levels of HNE and HNE-adducted proteins in the midbrain, and this could be directly correlated to a reduction in dopamine and metabolites in the striatum. Lastly, a recent study clearly demonstrates that intracerebral injection of HNE results in neurodegeneration.35 Therefore, HNE can impair key areas in the brain, leading to neuronal cell death. Carnosine, as a carbonyl scavenger and antioxidant, can provide protection against HNE and decrease neurodegenerative disorders,36 but its action is not limited to HNE-dependent damage.
Cardiovascular diseases
HNE has been shown to be implicated in cardiovascular diseases, as an accumulation of HNE was described in atherosclerotic lesions in both human and animals. LDLs can be oxidized by ROS from vascular cells. This leads to the formation of HNE and other aldehydes. HNE can form adducts with apoB. Oxidized-LDL bound to HNE-adducted apoB has a lower affinity for the apoB/E receptors that are expressed in most cell lines, except macrophages. Such modified LDLs are then reoriented toward scavenger receptors, expressed at the surface of macrophages and smooth muscle cells, leading to the formation of foam cells. The accumulation of foam cells promotes apoptosis induction and the formation of lipid cores.37 The subsequent atheromatous plaque formation involves macrophage infiltration and activation of smooth muscle cells leading to fibrogenesis. HNE can particularly form adducts with PDGFR (platelet-derived growth factor receptor) in atherosclerotic aortas and the use of the antioxidant hydralazine prevents HNE-related adduction and slows the progression of the disease.38
HNE could also promote chronic inflammation by stimulating the expression and the synthesis of MCP-1 (monocyte chemotactic protein 1) and TGFβ (transforming growth factor β) in macrophages and smooth muscle cells.39 Moreover, HNE could affect the vascular cells' barrier integrity, leading to apoptosis of endothelial cells.40, 41
HNE is also involved in myocardial infarction. After the ischemia/reperfusion sequence, ROS are produced and accumulated. They promote the generation of HNE, which can then disrupt the actin cytoskeleton, alter Ca2+ homeostasis and trigger cardiomyocyte cell death.42
Metabolic syndrome
Metabolic syndrome is a combination of metabolic disorders that may include impaired glucose tolerance, insulin resistance, dyslipidemia, obesity and liver disease. In diabetes, pancreatic β cells have been described to be highly sensitive to ROS. Therefore, HNE, which can trigger β cell apoptosis, may induce glucose intolerance and the development of diabetes.24
In non-alcoholic fatty liver disease (NAFLD), the persistent JNK (c-jun N-terminal kinase) activation by oxidative stress and HNE in hepatocytes induces cell death.43 In alcohol liver damage, protein modifications (adduction, haptenation) by aldehydes modify self-proteins and thus, stimulate the production of auto-antibodies and autoimmune reactions.2 More precisely, during the early phases of cirrhosis, antibodies against serum albumin adducted to MDA and HNE are detected in patients' sera. The antibody levels are higher in heavy drinkers with cirrhosis or extensive fibrosis than in those with fatty liver only. The formation of antigens derived from lipid peroxidation contributes to the development of immune responses associated with alcoholic liver disease.44 Finally, the importance of HNE in ethanol-induced steatosis was underlined by some studies relative to TNFα (tumor necrosis factor alpha)-induced apoptosis. Ethanol feeding appears to induce HNE-protein adducts, linked to an increase in TNFα secretion and apoptosis induction (TUNEL-caspase activation). When mice are coexposed to ethanol and antioxidants such N-acetyl-cysteine (NAC),45 the decrease in HNE-protein adducts protects hepatocytes against cell death.
When hepatotoxicity is induced by chemical treatments in vivo, such as acetaminophen, the supplementation with S-adenosylmethionine protects the liver, prevents lipoperoxidation and GSH (glutathione) depletion and decreases centrilobular necrosis.46
Cancer
Redox homeostasis appears to be modified in cancer cells.47 The activation of oncogenes, the modification of energy metabolism, the mitochondrial dysfunction and the inflammation in the surrounding tissues lead to the increase in oxidative stress during carcinogenesis. However, the level of lipid peroxidation products in cancer cells is still debated. On one hand, early studies have shown low HNE levels in tumor tissues compared with healthy tissues.48, 49, 50 Low levels of HNE have been shown to be linked with a decrease in TGFβ1, a cell growth inhibitory cytokine known to be downregulated in a large number of human malignant colon tumors. The decrease in TGFβ1 has been correlated with an increase in carcinogenesis progression.51 On the other hand, some studies have demonstrated increased HNE levels in cancer tissues,25, 52, 53 and the HNE-adduct levels seem to be positively correlated to progression in both the grade of malignancy in brain cancers54 and the stages of hepatitis.55 Several hypotheses can be formulated to explain these divergences. First, the considerable heterogeneity of tumor cells implies different patterns of the lipid composition of membranes, with an increase in cholesterol and a decrease in PUFA content and therefore, a lower formation of HNE.56 Moreover, tumor cells can exhibit higher expression of detoxification enzymes and antioxidant proteins that permit a better HNE extrusion.57 Finally, different grades of the tumors studied with a low lipoperoxydation level have also been related to better proliferative potential.58
In the case of the carcinogenic effect of HNE as an exogenous compound, during the promotion of colorectal carcinogenesis by heme iron, HNE has been shown to be produced in the colon lumen. It has been shown that normal cells are highly sensitive to HNE, whereas preneoplastic cells are resistant. Thus, it can promote the positive selection of preneoplastic cells, finally leading to the development of colorectal cancer.14, 59
As HNE can bind to guanine bases, it is mutagenic and genotoxic in vitro60 and that might contribute to cancer initiation in vivo.61 It may also contribute to cancer promotion by inhibiting DNA repair62 or by promoting inflammation.6
A strong induction of oxidative stress with HNE formation in advanced stage cancer cells using natural and chemical drugs is a therapeutic strategy to trigger apoptosis.47 However, the acquisition of high antioxidant defense by cancer cells can also be a limiting factor for radiotherapy and chemotherapy. The use of a combination of drugs that target cancer cells and break down their antioxidant defenses can be a promising strategy to specifically induce cell death in cancer cells.47
Diseases occurring due to oxidative stress notably involve cell death processes, such as apoptosis. Our goal here is to focus on the HNE regulation of cell death/survival, related to oxidative stress-dependent disorders. These relationships are complex. First, apoptosis can be mediated by different pathways and is not the only process of the induction of cell death. Second, HNE has dose-dependent effects, and the consequences on the cell are strongly modulated by the ability of the oxidative stress defenses to metabolize HNE. Lastly, based on the intrinsic nature of the cells, their responses regarding HNE could be multiple and in favor of the emergence of pathologies.
HNE: a Serial Killer
Apoptosis is a major pathway of cell death characterized by cell shrinkage, cell surface blebbing, chromatin condensation and DNA fragmentation. It is an active and programmed cell death that occurs via two main pathways: the extrinsic pathway, also called ‘death receptor pathway' and the intrinsic pathway or ‘mitochondrial pathway'. HNE can directly induce apoptosis but it can also be a mediator of apoptosis, as it can be generated by ROS (hydrogen peroxide), heat stress,63 UVA irradiations64 and pro-oxidant compounds like ethanol.65
HNE induces the extrinsic apoptotic pathway
The extrinsic apoptotic pathway transmits a death signal from the cell surface to the intracellular compartment and is initiated by the activation of death receptors like receptors of TNF, Fas/CD95 or TRAIL (TNF-related apoptosis-inducing ligand). Basically, the canonical signaling pathway involves the binding of their respective cytokines (TNFα, Fas Ligand (FasL), TRAIL), which triggers the aggregation of the receptors and the recruitment of adaptator proteins leading to the activation of initiator caspases (8 and 10). Executioner caspases (3 and 7) are then activated, promoting the cleavage of many substrates contributing to the execution of apoptosis, but the recruitment of the signaling proteins is dependent on the nature of the death receptor. As a relevant example for HNE-mediated apoptosis, the binding of FasL on its receptor Fas triggers its aggregation that leads to the formation of DISC (death-inducing signaling complex) involving the adaptator protein Fas-associated protein with death domain (FADD) and pro-caspase 8/10.66 The promiscuity of all these proteins triggers the activation and the autocatalytic cleavage of caspase 8/10. Consequently, the caspase-dependent cascade leads to apoptosis.
In the case of the impact of HNE on the extrinsic pathway, in the eye, it promotes the expression of Fas, allowing a sensitization of the lens epithelial cells to apoptosis when FasL is present in the microenvironment as a soluble cytokine or expressed on the neighboring in cells' membrane as a transmembrane form. The induction of Fas by HNE is associated with JNK activation and apoptosis induction67 but the modalities of Fas transcriptional regulation are still to be established. The links between HNE and Fas are of interest because they rely on physiological levels of HNE. One strategy to modulate basal HNE concentrations has been developed by the Awasthi's group, based on the overexpression or the downregulation of GSTA4, the main HNE-detoxifying enzyme. When human GSTA4 was overexpressed in lens epithelial cells, the levels of HNE decreased and Fas was strongly downregulated.68 Moreover, when GSTA4 was downregulated or invalidated in vivo in mice, the levels of HNE were increased68 and Fas expression was induced.67 The basal levels of HNE in the cell can then contribute to their sensitivity or their resistance regarding FasL stimulation or even HNE. For this latter point, it is worth noting that Fas-deficient lens epithelial cells are resistant to HNE -induced apoptosis according to the mechanism detailed below.
The main pathway of Fas-signaling activation by HNE appears to be DISC-independent67, 69 (without caspase 8 and FADD). Indeed, HNE can induce Fas-dependent apoptosis in pro-caspase 8-deficient Jurkat cells.69 The hypothetical mechanism can be dependent on the capacity of HNE to form protein adducts. The HNE-adduct formation with a membrane receptor could mimic ligand-cell surface receptor binding, which could then activate the related-signaling pathway. This model was proposed for EGFR (epidermal growth factor receptor) or PDGFR.23 Fas is a death receptor with a cysteine-enriched extracellular domain and HNE has been shown to form adducts with Fas in vitro.69 The binding of HNE on Fas seems primordial, as cell pretreatment with a Fas antagonizing antibody induces resistance to apoptosis triggered by HNE.
HNE activation of Fas without DISC involvement is enabled by the downstream activation of ASK1 (apoptosis signal-regulating kinase 1), JNK and caspase 3. Interestingly, HNE also activates a negative feedback on Fas activation, by a mechanism involving Daxx (death domain-associated protein). Daxx is a nuclear protein which is associated with DNA-binding transcription factors involved in stress response and known to repress their activities.70 After its export from the nucleus to the cytosol, it can interact with Fas. The pathway involving ASK1, JNK and caspase 3 is then inhibited and leads to the repression of apoptosis.71 Daxx silencing induces an exacerbation of the HNE-induced apoptosis suggesting the prominent role of Daxx as a negative regulator of apoptosis, thus avoiding massive cell death.69 In 2010, the Awasthi's team demonstrated that HNE induced both expression of Fas and Daxx. HNE can create covalent links with Daxx via some histidine residues, and in this way increase its export from the nucleus to the cytoplasm. The Fas-Daxx binding could have a negative effect on the ASK1-JNK signaling pathway and apoptosis induction. These mechanisms could be relevant for apoptosis induction by oxidative stress. If the stress is moderate, the couple Daxx-Fas limits apoptosis induction, whereas if the oxidative stress is massive, the pathway mediated by Fas-ASK1 and JNK is major and triggers massive cell death. Taking into account that HNE is diffusible, this negative loop can preserve the tissue integrity, as has been observed in vivo.72 This self-regulatory function for Daxx has also been described in Fas-dependent apoptosis after UV- and doxorubicin treatments, conditions that are commonly associated with oxidative stress. However, the role of Daxx as an apoptosis regulator is still controversial.70 On the contrary, some studies demonstrate that Daxx binding on Fas promotes cell death: the process being independent of DISC formation.73 The differences in Daxx activity as a pro- or an anti-apoptotic factor can arise from the fact that Daxx has a role both in the cytosol and in the nucleus. The complexity of its regulation can also depend on the cell types and the relative importance of Fas and JNK pathways. Lastly, contributing to the negative feedback of apoptosis regulation, it has also been shown that HNE treatment induces the downregulation of pro-apoptotic-associated genes such as IER3 (immediate early response 3), TRAF3 (tumor necrosis factor-a receptor-associated factor 3) in RKO human colorectal carcinoma cells,74 CAD (caspase-activated DNAse), FAST K (Fas-activated serine/threonine kinase) and DFF45 (DNA fragmentation factor 45) in ARPE-19 human retinal pigmental epithelium cell line.75
HNE-induced extrinsic apoptosis has been described in various cell models, and especially in human eye epithelial cell lines (HLE-B3 and ARPE-19) and leukemia cell lines (Jurkat, CRL2571). These models are very relevant from a clinical point of view. Retina is one of the organs most sensitive to HNE, first, because of the high consumption of oxygen by photoreceptors that generates high levels of ROS, and because its membranes contain one of the highest percentages of PUFAs. The pathways by which HNE induces apoptosis are relevant for understanding retinal damage and vision degradation in retinopathies.76 In the case of leukemia cells and HNE-induced extrinsic apoptosis, numerous studies have shown that patients at all stages of illness present an abnormally elevated oxidative status,77 whereas elevated ROS levels have been detected in chronic and acute myeloid malignancies. The efficacy of molecules with therapeutic potential, such as dithiolethione,78 was shown using HNE as a potent oxidative stress mediator in derived leukemia cell lines. These preventive strategies are based on HNE scavenging or on an improvement of cell detoxification regarding HNE (see last part of this review). The extrinsic apoptotic pathway mediated by HNE is summarized in Figure 2.
Figure 2.
Main extrinsic apoptotic pathway induced by HNE and Daxx-dependent-negative feedback. Extrinsic cell death can be mediated by Fas receptor, via the induction of Fas expression at the membrane or the direct binding of HNE on Fas, leading to its aggregation, independent on FasL and DISC formation. This latter process involves the ASK1/JNK pathway triggering AP-1-dependent transcription. In purple is represented the negative feedback with Daxx, modulating Fas-apoptotic signal. AP-1: activator protein 1; ASK1: apoptosis signal-regulating kinase 1; Daxx: death domain-associated protein; FADD: Fas-associated protein with death domain; FasL: Fas ligand; HNE: hydroxynonenal; and JNK: c-Jun N-terminal kinase
HNE induces the intrinsic apoptotic pathway
The intrinsic apoptotic pathway, also called the mitochondrial pathway, is the major apoptotic pathway highly conserved in vertebrates. Mitochondria have the capacity to integrate the pro-apoptotic signals from a large panel of extra- and intracellular stimuli (oxidative stress, starvation, radiation, DNA damage and toxins). These signals lead to mitochondrial outer membrane permeabilization (MOMP), which results in the release of different factors from the intermembrane space into the cytosol. Depending on the nature of the released pro-apoptotic factors, the mitochondria trigger caspase-dependent or caspase-independent apoptosis. The release of cytochrome c (cyt c) leads to the formation of the apoptosome, with the cytosolic proteins APAF1 (apoptosis protease-activating factor-1) and the initiator caspase 9. This complex initiates the autocatalytic cleavage of caspase 9, its activation and the subsequent cleavage of executioner caspases such as caspases 3, 6 and 7. In the cytosol, some endogenous caspase inhibitors like IAP (inhibitor of apoptosis protein) prevent caspase activation. The mitochondrial release of the proteins Smac/Diablo (second mitochondrial activator of caspases) and Omi/htrA2 prevents XIAP (X-linked IAP) activity and by this means mediates caspase activation. Mitochondria are also major actors in caspase-independent apoptosis. This process also involves MOMP but the release of killer proteins like AIF (apoptosis-inducing factor) and endonuclease G from the mitochondria to the nucleus triggers a caspase-independent DNA fragmentation.79
There are some examples showing that HNE can induce mitochondria-dependent apoptosis: mouse leukemic macrophage cell line80 exposed to HNE show the classical hallmarks of intrinsic apoptosis such as anti-apoptotic protein downregulation, pro-apoptotic protein upregulation, cyt c/AIF release and DNA fragmentation. During muscle cell apoptosis in age-related sarcopenia, the increase in HNE generation leads to anti-apoptotic protein inactivation and JNK and caspase 2/9 activation.81 The same observation was made in the RKO colon cancer cell line82 and in the PC12 neuronal cell line.83
The early events upstream of mitochondria targeting are diverse and are highly dependent on the initial stimulus. Cardiomyocytes treated with physiological concentrations of HNE show a progressive depletion in their mitochondrial bioenergetic reserve that leads to respiratory failure and cell death. This pathway is supposed to be important in myocardial pathologies where HNE could contribute to tissue damage by the increase in oxygen consumption, the subsequent mitochondrial failure and finally the depletion of energetic capacity.84 In cultured hippocampal neurons, HNE exposure can cause impairment of ATPase activities and a subsequent abnormal increase in [Ca2+]. This disruption acts as an apoptotic signal. Whatever the apoptotic stimulus, the Bcl2 (B-cell lymphoma 2) protein family has the role of an integration crossroad. Some of Bcl2 family members are pro-apoptotic: Bax, Bak and the BH3-only proteins like Bid; whereas others are anti-apoptotic such as Bcl2, Mcl1 or Bcl-xl. The balance between these pro-apoptotic and anti-apoptotic factors determines the fate of the cell. Upon a stress signal, the pro-apoptotic Bax and Bak proteins multimerize and are inserted into the mitochondrial outer membrane, forming pores at the origin of the MOMP. The phosphorylation of Bcl2 at its interaction site with the other members can modulate its activity. HNE has recently been shown to stabilize the interaction between Bcl2 and IKK (inhibitor of kappa B (IκB) kinase). The IKK is then able to phosphorylate Bcl2 on its critical site, thereby altering its anti-apoptotic function.85
Moreover, HNE can directly affect mitochondrial integrity. Mitochondrial membranes contain a critical phospholipid called cardiolipin. Cardiolipin oxidation by cyt c can produce HNE.86 It has been shown that cardiolipin oxidation is necessary for the subsequent steps of intrinsic apoptosis. It activates MOMP and enables the activation of apoptogenic Bcl2 proteins. Finally, HNE can directly affect the cellular redox status by depleting GSH, which can then induce a mitochondrial crisis with mitochondrial ROS production87 and subsequent activation of caspases.88 HNE-induced apoptosis can then be prevented by mitochondrial respiratory chain inhibitors such as rotenone or stigmatellin.89
The intrinsic apoptotic pathway activated by HNE is summarized in Figure 3.
Figure 3.
Intrinsic apoptotic pathway is induced by oxidative stress, p53 and mitochondrial membrane disturbances. cyt.c: cytochrome c; HNE: hydroxynonenal; and MOMP: mitochondrial outer membrane permeabilization
HNE and JNK activation during apoptosis
Depending on the cell type, HNE-induced apoptosis can involve both extrinsic and intrinsic pathways, but the respective contribution of the two pathways remains elusive. However, there is some evidence to show that strong JNK activation is a common step in HNE-induced programmed cell death.90
Generally, JNK is known to have a central role in both intrinsic and extrinsic pathways. This kinase belongs to the mitogen-activated protein kinases (MAP kinases) family and can be activated in response to diverse stimuli such as oxidative stress, lipopolysaccharides, TNFα or endoplasmic reticulum (ER) stress. After being activated by phosphorylation, JNK can translocate to the nucleus and then transactivate transcription factors (c-jun and c-myc) that regulate the expression of pro-apoptotic genes such as FasL, Bak and TNFα.91
HNE was shown to interact directly with JNK by forming adducts. This leads to nuclear translocation of JNK in human hepatic stellate cells.92 Moreover, HNE-induced apoptosis in neuronal PC12 cells is clearly mediated by the JNK pathway and not by other MAP kinases p38 or ERK pathways.93, 94 In sympathetic neurons, the specific neuron-associated JNK3 is activated during HNE-induced apoptosis. This is accompanied by c-jun phosphorylation and these effects can be suppressed in JNK3-deficient neurons.95 The pretreatment of leukemic cells with JNK inhibitor makes these cells resistant to HNE-induced apoptosis.69 Finally, well-known antioxidant molecules like resveratrol and piceatannol prevent HNE-induced apoptosis by blocking the JNK pathway and subsequently c-jun phosphorylation and AP-1 signaling.96, 97 Further experiments should be carried out in order to evaluate the role of JNK in HNE-induced apoptosis to clarify the orientation of the cell death pathway: extrinsic pathway by the regulation of c-jun/AP-1 signaling or intrinsic pathway by modulation of mitochondrial proteins.
HNE and p53: relationship between genotoxicity and cell death
Tumor protein 53 (p53) is a tumor suppressor and a transcription factor that regulates gene expression related to the cell cycle, DNA repair and apoptosis. p53 is activated upon DNA damage and oxidative stress to protect and repair the cell, but if the stress or damage reach a threshold, p53 triggers cell death.
Sharma et al.98 showed that exogenous HNE results in the phosphorylation of p53 (on Ser 15) and its nuclear translocation in retinal epithelial cells. Such activation is involved in apoptosis induction. On the other hand, the simple accumulation of p53 in HNE-treated mouse macrophages was not associated with apoptosis induction.80 As the degradation of p53 is proteasome-dependent and as HNE is able to inhibit proteasome activity,20 one may suggest that p53 accumulation is the result of defective proteolysis rather than an active stabilization of p53. Moreover, HNE is known to be genotoxic99, 100 and the activation of p53 upon HNE-mediated DNA damage appears relevant in a context of apoptosis, to trigger the expression of Bax, caspase 3 and inhibit Bcl2.
A recent study has examined the conformational modifications of p53 in NDD.101 It showed that the formation of HNE-p53 adducts did not affect the native conformation of p53, unlike nitration. The nitrated and so unfolded p53 directly decreases its pro-apoptotic activity and favors the survival of damaged neurons. Therefore, in this study, HNE adduction did not have a major impact on p53 function but could constitute an aggravating factor.
On the contrary, as p53 is a master regulator of the anti-oxidative response, it can directly act on HNE-induced toxicity: a study performed on p53 (−/−) mice brains showed that the lack of p53 reduced endogenous HNE levels and protein adduction.102 Consequently, p53 should be involved in the generation of HNE and this positive feedback can have a major role in NDD in which the levels of p53 bound to HNE are elevated and correlated to neuronal cell death.103
Other Pathways of Cell Death: One Killer, Several Weapons
HNE-induced apoptosis appears to be caspase-dependent. However, cells treated with caspase inhibitors such as z-VAD-FMK or DEVD-FMK do not present a completely rescued phenotype,104 suggesting the involvement of caspase-independent apoptotic mechanisms.
Calpain-mediated cell death
Intracellular Ca2+ levels are tightly regulated and Ca2+ is stored mainly in the ER and mitochondria under normal conditions. ROS attack can lead to the loss of ER membrane integrity, which is associated with a release of Ca2+ into the cytoplasm. Thus, lipid oxidation and notably through HNE, can be a signal for Ca2+ mobilization105 and subsequent calpain activation. Calpain is a Ca2+-dependent protease, located in the cytosol as an inactive precursor. In response to increased levels of cytosolic Ca2+, it translocates to the intracellular membranes and is activated by autocatalytic hydrolysis. Heat shock protein 70 (Hsp70), a chaperone protein and α-fodrin, a structural cystoskeletal protein, are known to be common substrates for calpains. Calpain-dependent Hsp70 cleavage is associated with lysosomal membrane permeabilization (LMP), which leads to the release of proteases called cathepsins into the cytoplasm, then triggering cell death. Actually, it has been shown that HNE induces α-fodrin cleavage106 and also the upregulation of Hsp70 expression and its carbonylation.69, 107 This post-translational modification is necessary for its cleavage by calpain, suggesting the participation of calpains in the cell death cascade.107 This link is still to be clearly established.
ER stress, proteasome inhibition and apoptosis induction
Because of its propensity to aggregate with proteins in the cell, HNE triggers the accumulation of protein aggregates in the cell that can overcome the proteasome. This can be amplified by a direct inhibition of proteolytic subunits: specific subunits of the 20S proteasome are targeted by HNE and the thus modified proteasome has impaired peptidase activity.20, 108
Moreover, ER stress and the activation of the unfolded protein response (UPR) are triggered when the ER is overwhelmed and cannot manage the processing and the folding of newly synthesized proteins and the elimination of unfolded or misfolded proteins. In atherosclerotic plaques, the protein modifications by LPO secondary products like aldehydes affect protein conformation and their activities. The accumulation of misfolded and undergraded proteins can lead to ER stress. If the ER stress is prolonged, it can turn into apoptosis induction. HNE was described as inducing ER stress and an UPR in human endothelial cells that had HNE-adducts with proteins colocalizing with ER.109 These adducted proteins directly affect ER function, especially protein disulfide isomerase.110 In support of this idea, it was shown that the folding of protein to allow optimal formation of disulfide bonds is highly redox-dependent in the ER.111 Thus, the accumulation of misfolded proteins with HNE-adducts in ER could compromise cell survival. The UPR is an adaptive process but the non-re-establishment of cell homeostasis strongly compromises tissue integrity and can initiate or promote pathologies.
Necrosis
To understand the effects of HNE, it is necessary to highlight the importance of the doses tested in each experiment. In the experiments of Chaudhary et al.107 on HepG2 cells exposed to 5–40 μM of HNE, the cells died by apoptosis, but the same cells exposed to 80–100 μM HNE underwent necrosis, a non-programmed and deleterious form of cell death in which organelles are damaged and the plasma membrane disrupted. Respectively 31.8% of the cell population that was exposed to 80 μM HNE and 55.4% for the 100 μM HNE treatment died by necrosis. Similarly, necrosis is induced by HNE at 100 μM in HeLa cells112 as well as in colorectal cell lines. All these in vitro data suggest a dose-dependent effect of HNE, with the induction of apoptosis at low doses and necrosis induction at high doses. The level of antioxidant defense has also to be taken into account (see chapter 7). However, because of the difficulty of quantifying free HNE in vivo, the data are limited concerning the possibility that such high concentrations of HNE could be reached in vivo and notably in oxidative stress-related pathologies.10
Iron-regulated cell death
Among the emerging cell death pathways closely related to HNE, iron-regulated cell death carves out a growing place. Ferritin is a protein involved in iron storage. The endocytosis of ferritin into lysosomes induces the release of free redox active Fe2+ which promotes ROS formation and subsequent lysosomal LPO. This generation of HNE leads to LMP. HNE-adducts were also detected in the cytosol, suggesting the spreading of LPO from the lysosome to the cytosol. Moderate LMP can result in apoptosis, whereas the total loss of lysosomal integrity triggers necrosis.113 However, the involvement of HNE in the balance of apoptosis/necrosis has to be reconsidered in the light of the recent studies on a new iron-regulated cell death mechanism called ferroptosis.114 This newly identified route of cell death is dependent not only on iron metabolism but also on fatty acid synthesis and cystine transport (involved in GSH synthesis). All these processes can be directly linked to HNE generation and to its detoxification-induced pathways. That is why further studies should be carried out to establish the importance of HNE in the cell sabotage leading to cell death.115
HNE Self Limits Apoptosis: Keeping Cool Under Pressure
Interestingly, HNE induces apoptosis but also initiates mechanisms that attenuate it. In respect to this latter aspect, the work by Jacobs and Marnett116 is particularly relevant. They demonstrated that the treatment of the RKO colorectal cancer cell line with HNE leads to a dose-dependent activation of HSF1 (heat shock factor 1), a transcription factor that protects cells against stress insults such as heat shock and oxidative stress. After HNE exposure, HSF1 is translocated from the cytoplasm to the nucleus and reduces HNE-induced apoptosis. The underlying mechanisms were identified by siRNA and overexpression, and they can be summed up as follow: first, HNE triggers the release of HSF1 from the regulator chaperones Hsp70 and Hsp90 (heat shock proteins), allowing its translocation to the nucleus. HSF1 activates the transcription of genes containing HSE (heat shock responsive element) via their promoters, such as Hsp40 and Hsp 70.1; the induced Hsp attenuates JNK-dependent apoptosis signaling and stabilizes Bcl-xL. The protective activation of HSF1 in HNE-induced apoptosis was also confirmed in HepG2 cells.107 Further experiments have demonstrated that the stabilization of Bcl2 anti-apoptotic proteins (like Mcl1, Bcl-xL and Bcl2) is dependent on the induction of BAG3 (Bcl2-associated athanogene domaine 3) that interacts with Hsp70 and Bcl-xL.117 The degree of protection gained by this pathway is thought to be superior to the protection afforded by the detoxification pathways like Nrf2.116
HNE Assault: The Choice to Stay Alive… Under Conditions
HNE as a cell cycle brake
The cell cycle is traditionally divided into four phases: G1, S, G2 and M, with a particular status for G1 cells, which can enter a resting state called G0. These quiescent cells represent the major part of the non-growing, non-proliferating cells in the organism. The transition from one cell cycle phase to another is regulated by CDK (cyclin-dependent kinase). Specific cyclins/CDK couples constitute checkpoints at each step of the cell cycle, and are influenced by pro-mitotic factors (like cdc25) and anti-mitotic factors (like cyclin-dependent kinases inhibitors). For an exhaustive review see Vermeulen et al.118
Several large scale studies performed using microarrays show that HNE is able to downregulate cell cycle promoting genes such as cell division cycle homologs (cdc20, cdc25), cyclins (A2, B1, B2, D1, F and K), topoisomerase II α, DNA polymerase δ, TGFα.74, 75 HNE also upregulates some cell cycle-arresting genes such as Gadd34 (growth arrest and DNA damage) or cyclin G2.74 However, the induction of cell cycle arrest by HNE has been known for a long time.119, 120 In HL60 cells, the way to limit HNE toxicity also seems to be based on the ability to stop cell cycle arrest in G0/G1 via the inhibition of cyclins D1, D2 and A and the hypophosphorylation of retinoblastoma protein (Rb). In 1998, Esterbauer et al.121 showed that treating the budding yeast Saccharomyces cerevisiae with HNE resulted in its temporary arrest in G1 phase. More recently, in prostate PC3 cells, HNE treatment was shown to trigger cell cycle arrest in G2/M, with dephosphorylation of cdc2.122 Finally, we have previously seen that HNE can self-limit its apoptosis by modulating Daxx activity.69 Moreover, Daxx is also known to interact with cell cycle regulatory proteins like HSF1, smad4 or p53.123 We can hypothesize that a Daxx-dependent-negative loop on HNE-induced apoptosis can also be dependent on the direct effect of Daxx on the cell cycle.
This ability of HNE to slow down cell proliferation at apoptosis-inducing concentrations can be interpreted as an alternative route to apoptosis, a possibility for slightly damaged cells attain self-rescue survival and for the tissue to maintain homeostasis in case of low grade injury.
Senescence
HNE-adducts are accumulated with aging in vivo and in vitro.124, 125 Senescence is a process associated with aging. Senescent cells are metabolically active cells in which growth is arrested in transition between the G1 and S phases. As senescent cells, these cells can no longer replicate, cannot re-enter the cell cycle and are characterized by shorter telomeres. Telomeres are repetitive sequences localized at the end of eukaryotic chromosomes. The synthesis of telomeric repeats is necessary for DNA replication and is ensured by a ribonucleoprotein called telomerase, only expressed in regenerative tissues. The length of telomeres decreases at each cell cycle division and constitutes a marker of somatic cell aging. Telomerase activity is reported to be reactivated during carcinogenesis and cancer cells escape senescence.126
It is well-described that oxidative stress accelerates telomere loss due to a decrease in telomerase activity in vitro.127 It has been reported that HNE (at non-lethal low doses) can downregulate expression of hTERT (human telomerase reverse transcriptase), the catalytic subunit of human telomerase, in leukemia cells and colon cancer cells. More precisely, HNE inhibits c-myc (an activator of the hTERT promoter) and activates Mad1 (a repressor of the hTERT promoter).128 Moreover, the activity of hTERT upon HNE exposure was also studied in endothelial cells isolated from patients with coronary artery disease. In this study, chronic treatment with NAC (an HNE scavenger and also a cysteine supplier for GSH synthesis129) led to a decrease in HNE levels and an activation of hTERT.125
Telomere shortening is a main cause of cellular senescence but very few studies have been done to characterize HNE-induced senescence, independently of its action on telomeres. This alternative pathway of senescence is characterized by the activity of β-galactosidase in the cytoplasm, the expression of cell cycle regulatory proteins p16 and p21 and the hypophosphorylation of Rb. Very recent experiments have characterized HNE-induced senescence in the development of atherosclerotic lesions in the blood vessels. HNE is secreted by activated macrophages and then induces senescence in vascular endothelial cells.130 Senescence will be of a great interest in the future as a major HNE-mediated aging mechanism.
Autophagy
Autophagy is a process that governs catabolic reactions and especially the clearance of long-lived proteins and organelles. It is characterized by autophagosome formation when the edges of the membrane initially fuse together to generate a large vacuole containing diverse macromolecules and cellular structures that will fuse with lysosomal membranes. This leads to the degradation of the contents of the autophagic vacuole.
After oxidative stress and HNE generation, the HNE-protein Michael-adducts are accumulated in the cell, and autophagy is a mechanisms by which such defective proteins can be degraded. In vascular smooth muscle cells (VSMC), HNE was shown to induce autophagy (50 μM for 30 min). This was characterized by cellular ultrastructural changes (identified by electron microscopy) and by the increase in LC-3-II levels (microtubule-associated protein 1 light chain 3 under the phosphatidylethanolamine-conjugated activated form).131 Such an induction could be dependent on mTOR inhibition through yet still unknown mechanisms. However, it appears that the inhibition of autophagy can trigger apoptosis, which supports the fact that autophagy is probably a defense system for survival.
Finally, the case of age-related macular degeneration is interesting because of the high specificity of the retinal pigment epithelium (RPE). This tissue is the site of high and permanent oxidative stress because the cells are prominent oxygen consumers and exposed to light. When LPO is high, it increases HNE adduction to proteins,132 among which are proteins of the photoreceptor outer segments (POS). HNE-adducted POS proteins are much less degraded by lysosomes and global proteolysis is decreased in RPE cells. Regarding that point, Krohne et al.133 have demonstrated that HNE-adducted POS directly reduces autophagy in RPE cells, leading to apoptosis induction.134
Therefore, VSMC exhibit a more adaptative capacity to survive HNE and oxidative stress than RPE cells.135 Retinal cells cannot sustain autophagy during massive LPO and finally die. In this context, autophagy is clearly a defense mechanism against HNE-protein adducts to maintain cell survival.
The Battle of Wills Between Detoxification and Death
For HNE, the balance between cell death and cell survival is largely dependent on the cells' abilities to cope with oxidative stress and especially to detoxify HNE.
Nrf2 and the antioxidant defense
Nrf2 is a master gene of antioxidant response. Under normal conditions, Nrf2 is in the cytosol, complexed with its inhibitor, Keap-1 (Kelch-like ECH-associated protein 1), which is an oxidant sensor. Cullin 3 ubiquitin ligase is attached to Keap-1 and is responsible for conjugating ubiquitin molecules to Nrf2, giving the signal to proteasome to degrade Nrf2 consecutively.136 In the presence of HNE, adducts on cysteines are formed in Keap-1 protein. This modifies the affinity of Keap-1 for Nrf2, which induces the release of Nrf2 and its export to the nucleus.137, 138 The nuclear Nrf2 recognizes consensus cis sequences called EpRE (electrophile response element) sequences. These sequences are present in the promoter region of genes coding for a great number of detoxification enzymes. Nrf2 can also form a heterodimer with c-jun and act independently or in synergy to activate antioxidant gene expression.139 The main genes which are regulated by Nrf2 are those that code for HO-1, NQO1, xCT (glutamate/cystine transporter), GCL, GSTs, ALDH, thioredoxin reductase 1 (Trx1) and AKR.136
Nrf2 is a major sensor of oxidative stress, and the disruption of Nrf2 abrogates antioxidant signaling, leading to the accumulation of HNE.140 Its activation is protective against oxidative stress and aging in vascular cells.141 However, the Nrf2 pathway is also a way for cancer cells to survive in a pro-oxidant environment57 and represents an important factor of resistance regarding chemotherapies in cancer.18, 142
Mechanisms of HNE detoxification
The half-life of HNE is less than 2 min. This short half-life underlines the importance of HNE detoxification as a cell defense system against HNE cytoxicity.
The key enzymes involved in HNE metabolism are GSTs, ALDHs and AKRs. GSTs have a major role in HNE detoxification:143 isoforms GSTA4–4 and GST5–8 have high catalyzing activity and allow the conjugation of HNE with the reduced form of gluthatione. This favors GSH-HNE export outside the cell via RLIP76 (RalA-binding protein 16 encoded 76-kDa splice variant) and/or MRPs (see Figure 4). The conjugation of HNE to GSH is primordial to neutralize HNE reactivity with proteins but also with DNA.
Figure 4.
Putative pathways involved in HNE detoxification. The conjugation of HNE with glutathione by GST is the main step of detoxification. The renewal of cellular GSH pool is dependent on cysteine content, regulation by xCT. The oxidation pathway via ALDHs family is supposed to be activated when stress is moderate. The reduction pathway with AKRs family is thought to be activated in case of acute stress. DHN: 1,4-dihydroxy-2-nonene; HNA: 4-hydroxynonenoic acid; AOR: aldehyde oxydoreductase; AKR: aldo-keto reductase; GST: glutathione S-transferase; ALDH: aldehyde dehydrogenase; xCT: cystine transporter; MRP: multidrug-resistant protein; RLIP76: RalA-binding protein 16 encoded 76-kDa splice variant; and Trx1: thioredoxine reductase 1
The GSH-HNE conjugates can be metabolized by two types of enzymes. The ALDH superfamily contributes to their oxidation in 4-hydroxynonenoïc acid (HNA)-lactone-GSH (HNA: 4-hydroxynonenoic acid), whereas the AKR superfamily contributes to their reduction in DHN-GSH (DHN: 1,4-dihydroxy-2-nonene). ALDHs have a prominent role. They can be downregulated in some disorders such as Parkinson disease. The recent study by Kong et al.144 shows that ALDH1A1 is a major HNE-detoxification enzyme in PC12 cells. Its overexpression results in reduced HNE toxicity, and its inhibition is neurotoxic, with a high accumulation of HNE-protein adducts. Other isoforms of ALDH, such as mitochondrial isoforms (ALDH5A and ALDH2), can also be determinant in HNE-mediated cell death. ALDH5A was described as metabolizing HNE in the brain.145 Finally, HNE itself can also be reduced by AOR (NAD(P)H-dependent alkenal/one oxidoreductase), and the generated metabolites (oxidized or not) can diffuse across the plasma membrane. xCT is the transporter subunit of the heterodimeric amino-acid transporter system xc-. It functions as an exchange transporter for cystine/glutamate, catalyzing the entry of cystine and the exit of glutamate at 1 : 1 ratio. Cystine is rapidly reduced by Trx into cysteine, which is the limiting substrate for GSH synthesis by γ-glutamylcysteine synthetase (γ-GCS) and GSH synthetase. Cells can also excrete cysteine to neutralize extracellular HNE by the formation of HNE-cysteine conjugates.14
The enzymes involved in HNE detoxification are essential in regulating its biological role, and it is worth noting that HNE can positively regulate its own metabolism by activating Nrf2. According to a consensus model, HNE consumes the bioavailable GSH in the cell during the detoxification process to form non-reactive GSH-HNE. The decrease in GSH levels in association with Nrf2 activation triggers GSH synthesis by a positive feedback mechanism.
Links between detoxification and cell death
The ability of HNE to form adducts with proteins is the major deleterious event it promotes. According to this, the extent of apoptosis is directly dependent on the yield of HNE-adducts that are accumulated in the cells.112 These effects are absent when cells are pretreated with the HNE scavenger NAC. NAC acts as a precursor of GSH synthesis as well as a stimulator of the cytosolic enzymes involved in GSH regeneration. It mediates protection by direct reaction between its reducing thiol groups and ROS.146 So a part of its anti-apoptotic activity could be due to its ability to modify the GSH/GSSG redox balance in the cell in favor of the reduced form. Indeed GSH/GSSG balance in favor of the oxidized species (that is, GSSG) constitutes an important signal that could decide the fate of a cell.147 However, a large number of HNE sequestering agents such carnosine or carnitine, act only by inhibiting the formation of HNE-protein adducts with a preferential formation of nontoxic scavenger-HNE adducts.
GSH conjugation is dependent on GST activity. The silencing of GSTA4-4 in human osteoarthritic chondrocytes increases HNE-induced cell death.148 On the contrary, HepG2 cells transfected with GSTA4-4 become resistant to HNE-induced apoptosis.149 The protective effect of GST overexpression against HNE-induced cell death has been observed in numerous cell lines (leukemia cells, retinal and lens epithelial cells). These cells acquire enhanced capacities to eliminate HNE, even after a prolonged treatment.
The entry of cystine, mediated by xCT, is also crucial for detoxification and cell death. Ferroptosis cell death is notably dependent on xCT inhibition and iron-mediated oxidative stress (see 4.4). The involvement of HNE in this death process has still to be determined.114 In 2006, Li et al.150 generated a murine transgenic cell line with stable expression of the AKR isoform AKR7A5. These cells were four times more resistant to HNE than the parental cell line. Similarly, Matsunaga et al.151 showed that stable transfection of AKR1C15 suppressed apoptosis induced by HNE in endothelial cells. All these data underline the importance of AKRs in the prevention of HNE-induced apoptosis.
Targeting the inhibition of ALDH isoforms also diminish the abilities of the cell to survive deleterious concentrations of HNE. On the contrary, the use of a pharmacological ALDH2 activator notably decreases HNE-adducts, prevents cell death and induces angiogenesis in human endothelial cells exposed to β-amyloid peptides.152 Finally, the cytosolic ALDH3A1 isoform, specialized in HNE-detoxification in the ocular tissue, ensures protection of the cornea in the case of UV-induced oxidative stress.153
Finally, it is worth noting that detoxification, like apoptosis, mobilizes energy. The intrinsic bioenergetic reserve capacity is determinant for the cellular response.84 That is why the basal level of antioxidant enzymes cannot be sufficient to tackle a chronic exposure to HNE. The induction of Nrf2 and the adaptability of the metabolic reserve directly impact on the cellular resistance regarding HNE assault. The new data about the involvement of Nrf2 in the metabolic reprogramming in cancer cells154 open new perspectives concerning the regulation of antioxidant defenses in response to HNE but also about the global fitness of the cell after HNE exposure.
Discussion
HNE is a ‘crossroads' molecule regulating signal transduction, gene expression, cell proliferation, stress-mediated signaling and cell death. However, the specificity of HNE is highly dependent on its concentration, the duration of exposure and the cell type. The threshold of oxidative stress tolerance is intrinsic to each cell type in a particular environment. At low concentrations (less than 5 μM), HNE has been reported to promote proliferation,155 and a silent stimulation of antioxidant responses by low levels of HNE (hormesis) can be protective72 against more drastic assaults such as by carcinogens.142 At higher concentrations (20–100 μM), HNE causes cell cycle arrest,120, 121, 122 disturbs differentiation156, 157 and triggers cell death. The modalities of apoptosis induction pathways are governed by the inherent nature of the cell, prone or not to ROS generation, the level of antioxidant defense and the induction of HNE metabolizing enzymes (summarized in Figure 5).
Figure 5.
Summary of cell fate upon HNE exposure. When a cell is exposed to a low dose of HNE, HNE can be removed by detoxification processes and the viability is not compromised. Upon a high dose of HNE, the response will depend on the capacity of the cell to eliminate HNE by detoxification. This capacity is controlled by three parameters: the cell type, the antioxidant defense (notably Nrf2) and the energetic adaptability. If the damages are important, the cells can only ‘subsist' by autophagy; senescence or cell cycle arrest. Otherwise, cell death is induced according to different pathways like apoptosis, necrosis or atypical cell death, sum up as ‘cell sabotage'. Chemopreventive strategies can directly target the detoxification process to eliminate HNE or directly prevent its formation. Nrf2: nuclear factor (erythroid-derived 2)-like 2
However, precise methods for quantifying HNE are lacking in vivo. HNE is a highly reactive aldehyde and because of the multiplicity of its metabolites and adducts, we get just a trace of its presence and not the real value of its concentration in a free-reactive state. As an example, the in vitro data obtained in leukemia cell lines show that 15-fold higher concentrations of HNE are required to induce apoptosis in primary leukocytes.158 We can conclude that primary cells are more resistant to HNE but we can also speculate as to whether the methods of cell culture modify the intrinsic antioxidant responses and the sensitivity toward HNE.
The pleiotropy of HNE targets (death receptors, mitochondria, p53 and transcription factors) makes it a real cell signaling molecule. Moreover, its half-life is longer than ROS such as H2O2 or O2·−; HNE is therefore a good signal propagator that leads to the cellular sabotage, fatal for cells. Interestingly, physiological levels of HNE are also able to affect the expression of genes involved in cell adhesion, cell cycle control, proliferation, cell growth and apoptosis.19 Thus, we should consider not only intracellular HNE concentrations after diverse stimuli/exposure but also the basal level inherent to the cell.
To go further down this road: under physiological conditions, linked to oxidative stress, HNE levels have been shown to positively correlate with aging, and may be responsible for various aging-related diseases (cancer, NDD, macular degenerescence and atherosclerosis) through DNA and protein damage. Therefore, HNE is a key target for the chemoprevention of these pathologies. Several strategies are possible: decreasing the exposure to pro-oxidant inducers, quenching the HNE-generating ROS or increasing the detoxification capacity of the cells. The overexpression of SOD (superoxide dismutase) or catalase, the use of natural antioxidants (vitamin C, vitamin E and β-carotene) or an increase in GSH synthesis have positive impacts on cellular protection. The improvement of cellular defenses is a relevant topic, with the use of natural compounds such as curcumin, sulforaphane, resveratrol or with chemical compounds such as tBHQ (tert-butylhydroquinone). They notably target the Nrf2 pathway.
To extend lifespan, a decrease in oxidative stress is recommended, by improving ROS and LPO sensing and maximizing the cellular antioxidant defenses.159 High-calorie diets (high fat and high carbohydrates) are linked to a high oxidant status in the organism, and recommended calorie restriction involves a decrease in total caloric intake while maintaining adequate nutrition, to extend the lifespan or improve health. Some enzymes, like HSP70, HO-1 or TRX1, involved in HNE detoxification belong to the vitagenes, a group of genes involved in the adaptation to oxidative stress and allow a greater resistance to long-lived animals.159 They are also regulated by Nrf2, which appears as a key protein in longevity.160 However, a chronic low level of HNE appears to be positive for longevity by continuously stimulating Nrf2 and antioxidant defense.72 Therefore, the links between HNE, detoxification pathways and diseases constitute a vast area of research for the understanding and the prevention of the major diseases of public health.
Acknowledgments
We thank Dr. Cecile Héliés-Toussaint, Dr. Dominique Lagadic-Gossmann, Dr. Anthony Lemarié, Dr. Fabrice Pierre, Dr. Sylviane Tâché and John Woodley for critical reading and English corrections of the manuscript. Research in authors' laboratory is supported by grants from the Agence Nationale pour la Recherche (ANR SecuriViande); Ligue Régionale Midi-Pyrénées (contre le Cancer) and Institut National du Cancer (INCA NeoMeaTox).
Glossary
- AIF
apoptosis-inducing factor
- AKR
aldo-keto reductase
- ALDH
aldehyde dehydrogenase
- ALE
advanced lipid peroxidation end product
- AP-1
activator protein 1
- ASK1
apoptosis signal-regulating kinase 1
- Bcl2
B-cell lymphoma 2
- Cdc
cell division cycle homolog
- CDK
cyclin-dependent kinase
- cyt.c
cytochrome c
- Daxx
death domain-associated protein
- DHN
1,4-dihydroxy-2-nonene
- DISC
death-inducing signaling complex
- ER
endoplasmic reticulum
- FADD
Fas-associated protein with death domain
- FasL
Fas ligand
- GSH
glutathione
- GST
gluthatione S-transferase
- HNA
4-hydroxynonenoïc acid
- HNE
4-hydroxy-2-nonenal
- HO-1
heme oxygenase 1
- HSF
heat shock factor
- Hsp
heat shock protein
- hTERT
human telomerase reverse transcriptase
- IκB
inhibitor of kappa B
- IKK
IκB kinase
- JNK
c-Jun N-terminal kinase
- Keap-1
Kelch-like ECH-associated protein 1
- LDL
low-density lipoprotein
- LMP
lysosomal membrane permeabilization
- LPO
lipoperoxidation
- MCP-1
monocyte chemotactic protein 1
- MOMP
mitochondrial outer membrane permeabilization
- MRP
multidrug-resistant protein
- NAFLD
non-alcoholic fatty liver disease
- NDD
neurodegenerative diseases
- NFκB
nuclear factor-kappa B
- NQO1
NAD(P)H dehydrogenase (quinone) 1
- Nrf2
Nuclear factor (erythroid-derived 2)-like 2
- p53
tumor protein 53
- PDGFR
platelet-derived growth factor receptor
- PUFA
polyunsaturated fatty acid
- Rb
retinoblastoma protein
- RLIP76
RalA-binding protein 16 encoded 76-kDa splice variant
- ROS
reactive oxygen species
- TGFβ
transforming growth factor β
- Trx1
thioredoxin reductase 1
- TNF
tumor necrosis factor
- TRAIL
TNF-related apoptosis-inducing ligand
- UPR
unfolded protein response
- VSMC
vascular smooth muscle cells
- XIAP
X-linked inhibitor of apoptosis protein
The authors declare no conflict of interest.
Footnotes
Edited by RA Knight
References
- Schaur RJ. Basic aspects of the biochemical reactivity of 4-hydroxynonenal. Mol Aspects Med. 2003;24:149–159. doi: 10.1016/s0098-2997(03)00009-8. [DOI] [PubMed] [Google Scholar]
- Duryee MJ, Willis MS, Freeman TL, Kuszynski CA, Tuma DJ, Klassen LW, et al. Mechanisms of alcohol liver damage: aldehydes, scavenger receptors, and autoimmunity. Front Bio sci. 2004;9:3145–3155. doi: 10.2741/1467. [DOI] [PubMed] [Google Scholar]
- Poli G, Schaur RJ, Siems WG, Leonarduzzi G. 4-hydroxynonenal: a membrane lipid oxidation product of medicinal interest. Med Res Rev. 2008;28:569–631. doi: 10.1002/med.20117. [DOI] [PubMed] [Google Scholar]
- Uchida K, Toyokuni S, Nishikawa K, Kawakishi S, Oda H, Hiai H, et al. Michael addition-type 4-hydroxy-2-nonenal adducts in modified low-density lipoproteins: markers for atherosclerosis. Biochemistry. 1994;33:12487–12494. doi: 10.1021/bi00207a016. [DOI] [PubMed] [Google Scholar]
- Pierre F, Peiro G, Tache S, Cross AJ, Bingham SA, Gasc N, et al. New marker of colon cancer risk associated with heme intake: 1,4-dihydroxynonane mercapturic acid. Cancer Epidemiol Biomarkers Prev. 2006;15:2274–2279. doi: 10.1158/1055-9965.EPI-06-0085. [DOI] [PubMed] [Google Scholar]
- Wang X, Yang Y, Moore DR, Nimmo SL, Lightfoot SA, Huycke MM. 4-hydroxy-2-nonenal mediates genotoxicity and bystander effects caused by Enterococcus faecalis-infected macrophages. Gastroenterology. 2012;142:543–551 e7. doi: 10.1053/j.gastro.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Parola M, Bellomo G, Robino G, Barrera G, Dianzani MU. 4-Hydroxynonenal as a biological signal: molecular basis and pathophysiological implications. Antioxid Redox Signal. 1999;1:255–284. doi: 10.1089/ars.1999.1.3-255. [DOI] [PubMed] [Google Scholar]
- Dwivedi S, Sharma A, Patrick B, Sharma R, Awasthi YC. Role of 4-hydroxynonenal and its metabolites in signaling. Redox Rep. 2007;12:4–10. doi: 10.1179/135100007X162211. [DOI] [PubMed] [Google Scholar]
- Smathers RL, Fritz KS, Galligan JJ, Shearn CT, Reigan P, Marks MJ, et al. Characterization of 4-HNE modified L-FABP reveals alterations in structural and functional dynamics. PLoS One. 2012;7:e38459. doi: 10.1371/journal.pone.0038459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grune T, Michel P, Sitte N, Eggert W, Albrecht-Nebe H, Esterbauer H, et al. Increased levels of 4-hydroxynonenal modified proteins in plasma of children with autoimmune diseases. Free Radic Biol Med. 1997;23:357–360. doi: 10.1016/s0891-5849(96)00586-2. [DOI] [PubMed] [Google Scholar]
- Siems WG, Brenke R, Beier A, Grune T. Oxidative stress in chronic lymphoedema. QJM. 2002;95:803–809. doi: 10.1093/qjmed/95.12.803. [DOI] [PubMed] [Google Scholar]
- Siems W, Grune T. Intracellular metabolism of 4-hydroxynonenal. Mol Aspects Med. 2003;24:167–175. doi: 10.1016/s0098-2997(03)00011-6. [DOI] [PubMed] [Google Scholar]
- Baradat M, Jouanin I, Dalleau S, Tache S, Gieules M, Debrauwer L, et al. 4-Hydroxy-2(E)-nonenal metabolism differs in Apc(+/+) cells and in Apc(Min/+) cells: it may explain colon cancer promotion by heme iron. Chem Res Toxicol. 2011;24:1984–1993. doi: 10.1021/tx2003036. [DOI] [PubMed] [Google Scholar]
- Siems WG, Zollner H, Grune T, Esterbauer H. Metabolic fate of 4-hydroxynonenal in hepatocytes: 1,4-dihydroxynonene is not the main product. J Lipid Res. 1997;38:612–622. [PubMed] [Google Scholar]
- Chiarpotto E, Domenicotti C, Paola D, Vitali A, Nitti M, Pronzato MA, et al. Regulation of rat hepatocyte protein kinase C beta isoenzymes by the lipid peroxidation product 4-hydroxy-2,3-nonenal: A signaling pathway to modulate vesicular transport of glycoproteins. Hepatology. 1999;29:1565–1572. doi: 10.1002/hep.510290510. [DOI] [PubMed] [Google Scholar]
- Zhang H, Forman HJ. Signaling pathways involved in phase II gene induction by alpha, beta-unsaturated aldehydes. Toxicol Ind Health. 2009;25:269–278. doi: 10.1177/0748233709102209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettazzoni P, Ciamporcero E, Medana C, Pizzimenti S, Dal Bello F, Minero VG, et al. Nuclear factor erythroid 2-related factor-2 activity controls 4-hydroxynonenal metabolism and activity in prostate cancer cells. Free Radic Biol Med. 2011;51:1610–1618. doi: 10.1016/j.freeradbiomed.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Patrick B, Li J, Jeyabal PV, Reddy PM, Yang Y, Sharma R, et al. Depletion of 4-hydroxynonenal in hGSTA4-transfected HLE B-3 cells results in profound changes in gene expression. Biochem Biophys Res Commun. 2005;334:425–432. doi: 10.1016/j.bbrc.2005.06.099. [DOI] [PubMed] [Google Scholar]
- Grune T, Davies KJ. The proteasomal system and HNE-modified proteins. Mol Aspects Med. 2003;24:195–204. doi: 10.1016/s0098-2997(03)00014-1. [DOI] [PubMed] [Google Scholar]
- Zarkovic K. 4-hydroxynonenal and neurodegenerative diseases. Mol Aspects Med. 2003;24:293–303. doi: 10.1016/s0098-2997(03)00024-4. [DOI] [PubMed] [Google Scholar]
- Choudhary S, Xiao T, Srivastava S, Zhang W, Chan LL, Vergara LA, et al. Toxicity and detoxification of lipid-derived aldehydes in cultured retinal pigmented epithelial cells. Toxicol Appl Pharmacol. 2005;204:122–134. doi: 10.1016/j.taap.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Negre-Salvayre A, Vieira O, Escargueil-Blanc I, Salvayre R. Oxidized LDL and 4-hydroxynonenal modulate tyrosine kinase receptor activity. Mol Aspects Med. 2003;24:251–261. doi: 10.1016/s0098-2997(03)00020-7. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Roles of the lipid peroxidation product 4-hydroxynonenal in obesity, the metabolic syndrome, and associated vascular and neurodegenerative disorders. Exp Gerontol. 2009;44:625–633. doi: 10.1016/j.exger.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karihtala P, Kauppila S, Puistola U, Jukkola-Vuorinen A. Divergent behaviour of oxidative stress markers 8-hydroxydeoxyguanosine (8-OHdG) and 4-hydroxy-2-nonenal (HNE) in breast carcinogenesis. Histopathology. 2011;58:854–862. doi: 10.1111/j.1365-2559.2011.03835.x. [DOI] [PubMed] [Google Scholar]
- Perry EA, Castellani RJ, Moreira PI, Nunomura A, Lui Q, Harris PL, et al. Neurofilaments are the major neuronal target of hydroxynonenal-mediated protein cross-links. Free Radic Res. 2013;47:507–510. doi: 10.3109/10715762.2013.794265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Swomley AM, Sultana R. Amyloid beta-peptide (1-42)-induced oxidative stress in alzheimer disease: importance in disease pathogenesis and progression. Antioxid Redox Signal. 2013;19:823–835. doi: 10.1089/ars.2012.5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shringarpure R, Grune T, Sitte N, Davies KJ. 4-Hydroxynonenal-modified amyloid-beta peptide inhibits the proteasome: possible importance in Alzheimer's disease. Cell Mol Life Sci. 2000;57:1802–1809. doi: 10.1007/PL00000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwell P, Yuan H, Magnusson R, Figueiredo-Pereira ME. Proteasome inhibition in neuronal cells induces a proinflammatory response manifested by upregulation of cyclooxygenase-2, its accumulation as ubiquitin conjugates, and production of the prostaglandin PGE(2) Arch Biochem Biophys. 2000;374:325–333. doi: 10.1006/abbi.1999.1646. [DOI] [PubMed] [Google Scholar]
- Keller JN, Pang Z, Geddes JW, Begley JG, Germeyer A, Waeg G, et al. Impairment of glucose and glutamate transport and induction of mitochondrial oxidative stress and dysfunction in synaptosomes by amyloid beta-peptide: role of the lipid peroxidation product 4-hydroxynonenal. J Neurochem. 1997;69:273–284. doi: 10.1046/j.1471-4159.1997.69010273.x. [DOI] [PubMed] [Google Scholar]
- Mark RJ, Lovell MA, Markesbery WR, Uchida K, Mattson MP. A role for 4-hydroxynonenal, an aldehydic product of lipid peroxidation, in disruption of ion homeostasis and neuronal death induced by amyloid beta-peptide. J Neurochem. 1997;68:255–264. doi: 10.1046/j.1471-4159.1997.68010255.x. [DOI] [PubMed] [Google Scholar]
- Jenner P.Oxidative stress in Parkinson's disease Ann Neurol 200353(Suppl 3S26–S36.discussion S36-8. [DOI] [PubMed] [Google Scholar]
- Morel P, Tallineau C, Pontcharraud R, Piriou A, Huguet F. Effects of 4-hydroxynonenal, a lipid peroxidation product, on dopamine transport and Na+/K+ ATPase in rat striatal synaptosomes. Neurochem Int. 1998;33:531–540. doi: 10.1016/s0197-0186(98)00062-x. [DOI] [PubMed] [Google Scholar]
- Westerlund M, Galter D, Carmine A, Olson L. Tissue- and species-specific expression patterns of class I, III, and IV Adh and Aldh 1 mRNAs in rodent embryos. Cell Tissue Res. 2005;322:227–236. doi: 10.1007/s00441-005-0038-7. [DOI] [PubMed] [Google Scholar]
- Al Nimer F, Strom M, Lindblom R, Aeinehband S, Bellander BM, Nyengaard JR, et al. Naturally occurring variation in the glutathione-s-transferase 4 gene determines neurodegeneration after traumatic brain injury. Antioxid Redox Signal. 2013;18:784–794. doi: 10.1089/ars.2011.4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipkiss AR. Carnosine and its possible roles in nutrition and health. Adv Food Nutr Res. 2009;57:87–154. doi: 10.1016/S1043-4526(09)57003-9. [DOI] [PubMed] [Google Scholar]
- Hegyi L, Skepper JN, Cary NR, Mitchinson MJ. Foam cell apoptosis and the development of the lipid core of human atherosclerosis. J Pathol. 1996;180:423–429. doi: 10.1002/(SICI)1096-9896(199612)180:4<423::AID-PATH677>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Vindis C, Escargueil-Blanc I, Elbaz M, Marcheix B, Grazide MH, Uchida K, et al. Desensitization of platelet-derived growth factor receptor-beta by oxidized lipids in vascular cells and atherosclerotic lesions: prevention by aldehyde scavengers. Circ Res. 2006;98:785–792. doi: 10.1161/01.RES.0000216288.93234.c3. [DOI] [PubMed] [Google Scholar]
- Leonarduzzi G, Chiarpotto E, Biasi F, Poli G. 4-Hydroxynonenal and cholesterol oxidation products in atherosclerosis. Mol Nutr Food Res. 2005;49:1044–1049. doi: 10.1002/mnfr.200500090. [DOI] [PubMed] [Google Scholar]
- Herbst U, Toborek M, Kaiser S, Mattson MP, Hennig B. 4-Hydroxynonenal induces dysfunction and apoptosis of cultured endothelial cells. J Cell Physiol. 1999;181:295–303. doi: 10.1002/(SICI)1097-4652(199911)181:2<295::AID-JCP11>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Hajjar DP, Haberland ME. Lipoprotein trafficking in vascular cells. Molecular Trojan horses and cellular saboteurs. J Biol Chem. 1997;272:22975–22978. doi: 10.1074/jbc.272.37.22975. [DOI] [PubMed] [Google Scholar]
- VanWinkle WB, Snuggs M, Miller JC, Buja LM. Cytoskeletal alterations in cultured cardiomyocytes following exposure to the lipid peroxidation product, 4-hydroxynonenal. Cell Motil Cytoskeleton. 1994;28:119–134. doi: 10.1002/cm.970280204. [DOI] [PubMed] [Google Scholar]
- Singh R, Wang Y, Schattenberg JM, Xiang Y, Czaja MJ. Chronic oxidative stress sensitizes hepatocytes to death from 4-hydroxynonenal by JNK/c-Jun overactivation. Am J Physiol Gastrointest Liver Physiol. 2009;297:G907–G917. doi: 10.1152/ajpgi.00151.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottaran E, Stewart SF, Rolla R, Vay D, Cipriani V, Moretti M, et al. Lipid peroxidation contributes to immune reactions associated with alcoholic liver disease. Free Radic Biol Med. 2002;32:38–45. doi: 10.1016/s0891-5849(01)00757-2. [DOI] [PubMed] [Google Scholar]
- Dou X, Li S, Wang Z, Gu D, Shen C, Yao T, et al. Inhibition of NF-kappaB activation by 4-hydroxynonenal contributes to liver injury in a mouse model of alcoholic liver disease. Am J Pathol. 2012;181:1702–1710. doi: 10.1016/j.ajpath.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentovic M, Terneus M, Harmon RC, Carpenter AB. S-Adenosylmethionine (SAMe) attenuates acetaminophen hepatotoxicity in C57BL/6 mice. Toxicol Lett. 2004;154:165–174. doi: 10.1016/j.toxlet.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Barrera G. Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol. 2012;2012:137289. doi: 10.5402/2012/137289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman KH, Burton GW, Ingold KU, Slater TF. Lipid peroxidation and lipid antioxidants in normal and tumor cells. Toxicol Pathol. 1984;12:235–239. doi: 10.1177/019262338401200305. [DOI] [PubMed] [Google Scholar]
- Tjalkens RB, Cook LW, Petersen DR. Formation and export of the glutathione conjugate of 4-hydroxy-2, 3-E-nonenal (4-HNE) in hepatoma cells. Arch Biochem Biophys. 1999;361:113–119. doi: 10.1006/abbi.1998.0946. [DOI] [PubMed] [Google Scholar]
- Oberley TD, Toyokuni S, Szweda LI. Localization of hydroxynonenal protein adducts in normal human kidney and selected human kidney cancers. Free Radic Biol Med. 1999;27:695–703. doi: 10.1016/s0891-5849(99)00117-3. [DOI] [PubMed] [Google Scholar]
- Biasi F, Tessitore L, Zanetti D, Cutrin JC, Zingaro B, Chiarpotto E, et al. Associated changes of lipid peroxidation and transforming growth factor beta1 levels in human colon cancer during tumour progression. Gut. 2002;50:361–367. doi: 10.1136/gut.50.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrzydlewska E, Stankiewicz A, Sulkowska M, Sulkowski S, Kasacka I. Antioxidant status and lipid peroxidation in colorectal cancer. J Toxicol Environ Health A. 2001;64:213–222. doi: 10.1080/15287390152543690. [DOI] [PubMed] [Google Scholar]
- Young O, Crotty T, O'Connell R, O'Sullivan J, Curran AJ. Levels of oxidative damage and lipid peroxidation in thyroid neoplasia. Head Neck. 2010;32:750–756. doi: 10.1002/hed.21247. [DOI] [PubMed] [Google Scholar]
- Juric-Sekhar G, Zarkovic K, Waeg G, Cipak A, Zarkovic N. Distribution of 4-hydroxynonenal-protein conjugates as a marker of lipid peroxidation and parameter of malignancy in astrocytic and ependymal tumors of the brain. Tumori. 2009;95:762–768. doi: 10.1177/030089160909500620. [DOI] [PubMed] [Google Scholar]
- Marquez-Quinones A, Cipak A, Zarkovic K, Fattel-Fazenda S, Villa-Trevino S, Waeg G, et al. HNE-protein adducts formation in different pre-carcinogenic stages of hepatitis in LEC rats. Free Radic Res. 2010;44:119–127. doi: 10.3109/10715760903338071. [DOI] [PubMed] [Google Scholar]
- Dianzani MU. Lipid peroxidation and cancer: a critical reconsideration. Tumori. 1989;75:351–357. doi: 10.1177/030089168907500410. [DOI] [PubMed] [Google Scholar]
- DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dianzani MU. Lipid peroxidation and cancer. Crit Rev Oncol Hematol. 1993;15:125–147. doi: 10.1016/1040-8428(93)90052-6. [DOI] [PubMed] [Google Scholar]
- Pierre F, Tache S, Gueraud F, Rerole AL, Jourdan ML, Petit C. Apc mutation induces resistance of colonic cells to lipoperoxide-triggered apoptosis induced by faecal water from haem-fed rats. Carcinogenesis. 2007;28:321–327. doi: 10.1093/carcin/bgl127. [DOI] [PubMed] [Google Scholar]
- Feng Z, Hu W, Amin S, Tang MS. Mutational spectrum and genotoxicity of the major lipid peroxidation product, trans-4-hydroxy-2-nonenal, induced DNA adducts in nucleotide excision repair-proficient and -deficient human cells. Biochemistry. 2003;42:7848–7854. doi: 10.1021/bi034431g. [DOI] [PubMed] [Google Scholar]
- Hu W, Feng Z, Eveleigh J, Iyer G, Pan J, Amin S, et al. The major lipid peroxidation product, trans-4-hydroxy-2-nonenal, preferentially forms DNA adducts at codon 249 of human p53 gene, a unique mutational hotspot in hepatocellular carcinoma. Carcinogenesis. 2002;23:1781–1789. doi: 10.1093/carcin/23.11.1781. [DOI] [PubMed] [Google Scholar]
- Feng Z, Hu W, Tang MS. Trans-4-hydroxy-2-nonenal inhibits nucleotide excision repair in human cells: a possible mechanism for lipid peroxidation-induced carcinogenesis. Proc Natl Acad Sci USA. 2004;101:8598–8602. doi: 10.1073/pnas.0402794101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JZ, Sharma R, Yang Y, Singhal SS, Sharma A, Saini MK, et al. Accelerated metabolism and exclusion of 4-hydroxynonenal through induction of RLIP76 and hGST5.8 is an early adaptive response of cells to heat and oxidative stress. J Biol Chem. 2001;276:41213–41223. doi: 10.1074/jbc.M106838200. [DOI] [PubMed] [Google Scholar]
- Yang Y, Sharma A, Sharma R, Patrick B, Singhal SS, Zimniak P, et al. Cells preconditioned with mild, transient UVA irradiation acquire resistance to oxidative stress and UVA-induced apoptosis: role of 4-hydroxynonenal in UVA-mediated signaling for apoptosis. J Biol Chem. 2003;278:41380–41388. doi: 10.1074/jbc.M305766200. [DOI] [PubMed] [Google Scholar]
- Ramachandran V, Watts LT, Maffi SK, Chen J, Schenker S, Henderson G. Ethanol-induced oxidative stress precedes mitochondrially mediated apoptotic death of cultured fetal cortical neurons. J Neurosci Res. 2003;74:577–588. doi: 10.1002/jnr.10767. [DOI] [PubMed] [Google Scholar]
- Wallach D, Kang TB, Kovalenko A. The extrinsic cell death pathway and the elan mortel. Cell Death Differ. 2008;15:1533–1541. doi: 10.1038/cdd.2008.41. [DOI] [PubMed] [Google Scholar]
- Li J, Sharma R, Patrick B, Sharma A, Jeyabal PV, Reddy PM, et al. Regulation of CD95 (Fas) expression and Fas-mediated apoptotic signaling in HLE B-3 cells by 4-hydroxynonenal. Biochemistry. 2006;45:12253–12264. doi: 10.1021/bi060780+. [DOI] [PubMed] [Google Scholar]
- Engle MR, Singh SP, Czernik PJ, Gaddy D, Montague DC, Ceci JD, et al. Physiological role of mGSTA4-4, a glutathione S-transferase metabolizing 4-hydroxynonenal: generation and analysis of mGsta4 null mouse. Toxicol Appl Pharmacol. 2004;194:296–308. doi: 10.1016/j.taap.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Sharma R, Sharma A, Dwivedi S, Zimniak P, Awasthi S, Awasthi YC. 4-Hydroxynonenal self-limits fas-mediated DISC-independent apoptosis by promoting export of Daxx from the nucleus to the cytosol and its binding to Fas. Biochemistry. 2008;47:143–156. doi: 10.1021/bi701559f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomoni P, Khelifi AF. Daxx: death or survival protein. Trends Cell Biol. 2006;16:97–104. doi: 10.1016/j.tcb.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Yang X, Khosravi-Far R, Chang HY, Baltimore D. Daxx a novel Fas-binding protein that activates JNK and apoptosis. Cell. 1997;89:1067–1076. doi: 10.1016/s0092-8674(00)80294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SP, Niemczyk M, Saini D, Sadovov V, Zimniak L, Zimniak P. Disruption of the mGsta4 gene increases life span of C57BL mice. J Gerontol A Biol Sci Med Sci. 2010;65:14–23. doi: 10.1093/gerona/glp165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii S, Egan DA, Evans RA, Reed JC. Human Daxx regulates Fas-induced apoptosis from nuclear PML oncogenic domains (PODs) EMBO J. 1999;18:6037–6049. doi: 10.1093/emboj/18.21.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JD, Marnett LJ. Alterations in gene expression induced by the lipid peroxidation product, 4-hydroxy-2-nonenal. Chem Res Toxicol. 2005;18:1642–1653. doi: 10.1021/tx050211n. [DOI] [PubMed] [Google Scholar]
- Weigel AL, Handa JT, Hjelmeland LM. Microarray analysis of H2O2-, HNE-, or tBH-treated ARPE-19 cells. Free Radic Biol Med. 2002;33:1419–1432. doi: 10.1016/s0891-5849(02)01082-1. [DOI] [PubMed] [Google Scholar]
- Marchette LD, Wang H, Li F, Babizhayev MA, Kasus-Jacobi A. Carcinine has 4-hydroxynonenal scavenging property and neuroprotective effect in mouse retina. Invest Ophthalmol Vis Sci. 53:3572–3583. doi: 10.1167/iovs.11-9042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battisti V, Maders LD, Bagatini MD, Santos KF, Spanevello RM, Maldonado PA, et al. Measurement of oxidative stress and antioxidant status in acute lymphoblastic leukemia patients. Clin Biochem. 2008;41:511–518. doi: 10.1016/j.clinbiochem.2008.01.027. [DOI] [PubMed] [Google Scholar]
- Khanna S, Sen CK, Roy S, Christen MO, Packer L. Protective effects of anethole dithiolethione against oxidative stress-induced cytotoxicity in human Jurkat T cells. Biochem Pharmacol. 1998;56:61–69. doi: 10.1016/s0006-2952(98)00113-0. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Joza N, Tasdemir E, Maiuri MC, Hengartner M, Abrams JM, et al. No death without life: vital functions of apoptotic effectors. Cell Death Differ. 2008;15:1113–1123. doi: 10.1038/cdd.2008.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes RL, Brune B, Townsend AJ. Apoptosis in RAW 264.7 cells exposed to 4-hydroxy-2-nonenal: dependence on cytochrome C release but not p53 accumulation. Free Radic Biol Med. 2001;30:884–894. doi: 10.1016/s0891-5849(01)00476-2. [DOI] [PubMed] [Google Scholar]
- Braga M, Sinha Hikim AP, Datta S, Ferrini MG, Brown D, Kovacheva EL, et al. Involvement of oxidative stress and caspase 2-mediated intrinsic pathway signaling in age-related increase in muscle cell apoptosis in mice. Apoptosis. 2008;13:822–832. doi: 10.1007/s10495-008-0216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji C, Amarnath V, Pietenpol JA, Marnett LJ. 4-hydroxynonenal induces apoptosis via caspase-3 activation and cytochrome c release. Chem Res Toxicol. 2001;14:1090–1096. doi: 10.1021/tx000186f. [DOI] [PubMed] [Google Scholar]
- Siddiqui MA, Kumar V, Kashyap MP, Agarwal M, Singh AK, Khanna VK, et al. Short-term exposure of 4-hydroxynonenal induces mitochondria-mediated apoptosis in PC12 cells. Hum Exp Toxicol. 2012;31:336–345. doi: 10.1177/0960327111432500. [DOI] [PubMed] [Google Scholar]
- Hill BG, Dranka BP, Zou L, Chatham JC, Darley-Usmar VM. Importance of the bioenergetic reserve capacity in response to cardiomyocyte stress induced by 4-hydroxynonenal. Biochem J. 2009;424:99–107. doi: 10.1042/BJ20090934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodur C, Kutuk O, Tezil T, Basaga H. Inactivation of Bcl-2 through IkappaB kinase (IKK)-dependent phosphorylation mediates apoptosis upon exposure to 4-hydroxynonenal (HNE) J Cell Physiol. 2012;227:3556–3565. doi: 10.1002/jcp.24057. [DOI] [PubMed] [Google Scholar]
- Liu W, Porter NA, Schneider C, Brash AR, Yin H. Formation of 4-hydroxynonenal from cardiolipin oxidation: Intramolecular peroxyl radical addition and decomposition. Free Radic Biol Med. 2011;50:166–178. doi: 10.1016/j.freeradbiomed.2010.10.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza H, John A. 4-hydroxynonenal induces mitochondrial oxidative stress, apoptosis and expression of glutathione S-transferase A4-4 and cytochrome P450 2E1 in PC12 cells. Toxicol Appl Pharmacol. 2006;216:309–318. doi: 10.1016/j.taap.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Liu W, Kato M, Akhand AA, Hayakawa A, Suzuki H, Miyata T, et al. 4-hydroxynonenal induces a cellular redox status-related activation of the caspase cascade for apoptotic cell death. J Cell Sci. 2000;113 (Pt 4:635–641. doi: 10.1242/jcs.113.4.635. [DOI] [PubMed] [Google Scholar]
- Lee JY, Jung GY, Heo HJ, Yun MR, Park JY, Bae SS, et al. 4-Hydroxynonenal induces vascular smooth muscle cell apoptosis through mitochondrial generation of reactive oxygen species. Toxicol Lett. 2006;166:212–221. doi: 10.1016/j.toxlet.2006.07.305. [DOI] [PubMed] [Google Scholar]
- Uchida K, Shiraishi M, Naito Y, Torii Y, Nakamura Y, Osawa T. Activation of stress signaling pathways by the end product of lipid peroxidation. 4-hydroxy-2-nonenal is a potential inducer of intracellular peroxide production. J Biol Chem. 1999;274:2234–2242. doi: 10.1074/jbc.274.4.2234. [DOI] [PubMed] [Google Scholar]
- Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008;27:6245–6251. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parola M, Robino G, Marra F, Pinzani M, Bellomo G, Leonarduzzi G, et al. HNE interacts directly with JNK isoforms in human hepatic stellate cells. J Clin Invest. 1998;102:1942–1950. doi: 10.1172/JCI1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song BJ, Soh Y, Bae M, Pie J, Wan J, Jeong K. Apoptosis of PC12 cells by 4-hydroxy-2-nonenal is mediated through selective activation of the c-Jun N-terminal protein kinase pathway. Chem Biol Interact. 2001;130-132:943–954. doi: 10.1016/s0009-2797(00)00247-7. [DOI] [PubMed] [Google Scholar]
- Kutuk O, Basaga H. Apoptosis signalling by 4-hydroxynonenal: a role for JNK-c-Jun/AP-1 pathway. Redox Rep. 2007;12:30–34. doi: 10.1179/135100007X162329. [DOI] [PubMed] [Google Scholar]
- Bruckner SR, Estus S. JNK3 contributes to c-jun induction and apoptosis in 4-hydroxynonenal-treated sympathetic neurons. J Neurosci Res. 2002;70:665–670. doi: 10.1002/jnr.10437. [DOI] [PubMed] [Google Scholar]
- Kutuk O, Poli G, Basaga H. Resveratrol protects against 4-hydroxynonenal-induced apoptosis by blocking JNK and c-JUN/AP-1 signaling. Toxicol Sci. 2006;90:120–132. doi: 10.1093/toxsci/kfj055. [DOI] [PubMed] [Google Scholar]
- Jang YJ, Kim JE, Kang NJ, Lee KW, Lee HJ. Piceatannol attenuates 4-hydroxynonenal-induced apoptosis of PC12 cells by blocking activation of c-Jun N-terminal kinase. Ann N Y Acad Sci. 2009;1171:176–182. doi: 10.1111/j.1749-6632.2009.04727.x. [DOI] [PubMed] [Google Scholar]
- Sharma A, Sharma R, Chaudhary P, Vatsyayan R, Pearce V, Jeyabal PV, et al. 4-Hydroxynonenal induces p53-mediated apoptosis in retinal pigment epithelial cells. Arch Biochem Biophys. 2008;480:85–94. doi: 10.1016/j.abb.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll N, Ruhe C, Veeriah S, Sauer J, Glei M, Gallagher EP, et al. Genotoxicity of 4-hydroxy-2-nonenal in human colon tumor cells is associated with cellular levels of glutathione and the modulation of glutathione S-transferase A4 expression by butyrate. Toxicol Sci. 2005;86:27–35. doi: 10.1093/toxsci/kfi171. [DOI] [PubMed] [Google Scholar]
- Eckl PM. Genotoxicity of HNE. Mol Aspects Med. 2003;24:161–165. doi: 10.1016/s0098-2997(03)00010-4. [DOI] [PubMed] [Google Scholar]
- Buizza L, Prandelli C, Bonini SA, Delbarba A, Cenini G, Lanni C, et al. Conformational altered p53 affects neuronal function: relevance for the response to toxic insult and growth-associated protein 43 expression. Cell Death Dis. 2013;4:e484. doi: 10.1038/cddis.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone E, Cenini G, Sultana R, Di Domenico F, Fiorini A, Perluigi M, et al. Lack of p53 decreases basal oxidative stress levels in the brain through upregulation of thioredoxin-1, biliverdin reductase-A, manganese superoxide dismutase, and nuclear factor kappa-B. Antioxid Redox Signal. 2012;16:1407–1420. doi: 10.1089/ars.2011.4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenini G, Sultana R, Memo M, Butterfield DA. Elevated levels of pro-apoptotic p53 and its oxidative modification by the lipid peroxidation product, HNE, in brain from subjects with amnestic mild cognitive impairment and Alzheimer's disease. J Cell Mol Med. 2008;12:987–994. doi: 10.1111/j.1582-4934.2008.00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary S, Zhang W, Zhou F, Campbell GA, Chan LL, Thompson EB, et al. Cellular lipid peroxidation end-products induce apoptosis in human lens epithelial cells. Free Radic Biol Med. 2002;32:360–369. doi: 10.1016/s0891-5849(01)00810-3. [DOI] [PubMed] [Google Scholar]
- Kruman II, Mattson MP. Pivotal role of mitochondrial calcium uptake in neural cell apoptosis and necrosis. J Neurochem. 1999;72:529–540. doi: 10.1046/j.1471-4159.1999.0720529.x. [DOI] [PubMed] [Google Scholar]
- Peng ZF, Koh CH, Li QT, Manikandan J, Melendez AJ, Tang SY, et al. Deciphering the mechanism of HNE-induced apoptosis in cultured murine cortical neurons: transcriptional responses and cellular pathways. Neuropharmacology. 2007;53:687–698. doi: 10.1016/j.neuropharm.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Chaudhary P, Sharma R, Sharma A, Vatsyayan R, Yadav S, Singhal SS, et al. Mechanisms of 4-hydroxy-2-nonenal induced pro- and anti-apoptotic signaling. Biochemistry. 2010;49:6263–6275. doi: 10.1021/bi100517x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrington DA, Kapphahn RJ. Catalytic site-specific inhibition of the 20S proteasome by 4-hydroxynonenal. FEBS Lett. 2004;578:217–223. doi: 10.1016/j.febslet.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Vladykovskaya E, Sithu SD, Haberzettl P, Wickramasinghe NS, Merchant ML, Hill BG, et al. Lipid peroxidation product 4-hydroxy-trans-2-nonenal causes endothelial activation by inducing endoplasmic reticulum stress. J Biol Chem. 2012;287:11398–11409. doi: 10.1074/jbc.M111.320416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller C, Bandemer J, Vindis C, Camare C, Mucher E, Gueraud F, et al. Protein disulfide isomerase modification and inhibition contribute to er stress and apoptosis induced by oxidized low density lipoproteins. Antioxid Redox Signal. 2013;18:731–742. doi: 10.1089/ars.2012.4577. [DOI] [PubMed] [Google Scholar]
- Santos CX, Tanaka LY, Wosniak J, Laurindo FR. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid Redox Signal. 2009;11:2409–2427. doi: 10.1089/ars.2009.2625. [DOI] [PubMed] [Google Scholar]
- Sovic A, Borovic S, Loncaric I, Kreuzer T, Zarkovic K, Vukovic T, et al. The carcinostatic and proapoptotic potential of 4-hydroxynonenal in HeLa cells is associated with its conjugation to cellular proteins. Anticancer Res. 2001;21:1997–2004. [PubMed] [Google Scholar]
- Bresgen N, Jaksch H, Lacher H, Ohlenschlager I, Uchida K, Eckl PM. Iron mediated oxidative stress plays an essential role in ferritin induced cell death. Free Radic Biol Med. 2010;48:1347–1357. doi: 10.1016/j.freeradbiomed.2010.02.019. [DOI] [PubMed] [Google Scholar]
- Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR, Victor B. The pantheon of the fallen: why are there so many forms of cell death. Trends Cell Biol. 2012;22:555–556. doi: 10.1016/j.tcb.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs AT, Marnett LJ. Heat shock factor 1 attenuates 4-hydroxynonenal-mediated apoptosis: critical role for heat shock protein 70 induction and stabilization of Bcl-XL. J Biol Chem. 2007;282:33412–33420. doi: 10.1074/jbc.M706799200. [DOI] [PubMed] [Google Scholar]
- Jacobs AT, Marnett LJ. HSF1-mediated BAG3 expression attenuates apoptosis in 4-hydroxynonenal-treated colon cancer cells via stabilization of anti-apoptotic Bcl-2 proteins. J Biol Chem. 2009;284:9176–9183. doi: 10.1074/jbc.M808656200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen K, Van Bockstaele DR, Berneman ZN. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003;36:131–149. doi: 10.1046/j.1365-2184.2003.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera G, Pizzimenti S, Laurora S, Briatore F, Toaldo C, Dianzani MU. 4-hydroxynonenal and cell cycle. Biofactors. 2005;24:151–157. doi: 10.1002/biof.5520240118. [DOI] [PubMed] [Google Scholar]
- Pizzimenti S, Barrera G, Dianzani MU, Brusselbach S. Inhibition of D1, D2, and A-cyclin expression in HL-60 cells by the lipid peroxydation product 4-hydroxynonenal. Free Radic Biol Med. 1999;26:1578–1586. doi: 10.1016/s0891-5849(99)00022-2. [DOI] [PubMed] [Google Scholar]
- Wonisch W, Kohlwein SD, Schaur J, Tatzber F, Guttenberger H, Zarkovic N, et al. Treatment of the budding yeast Saccharomyces cerevisiae with the lipid peroxidation product 4-HNE provokes a temporary cell cycle arrest in G1 phase. Free Radic Biol Med. 1998;25:682–687. doi: 10.1016/s0891-5849(98)00110-5. [DOI] [PubMed] [Google Scholar]
- Pettazzoni P, Pizzimenti S, Toaldo C, Sotomayor P, Tagliavacca L, Liu S, et al. Induction of cell cycle arrest and DNA damage by the HDAC inhibitor panobinostat (LBH589) and the lipid peroxidation end product 4-hydroxynonenal in prostate cancer cells. Free Radic Biol Med. 50:313–322. doi: 10.1016/j.freeradbiomed.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Laurora S, Tamagno E, Briatore F, Bardini P, Pizzimenti S, Toaldo C, et al. 4-Hydroxynonenal modulation of p53 family gene expression in the SK-N-BE neuroblastoma cell line. Free Radic Biol Med. 2005;38:215–225. doi: 10.1016/j.freeradbiomed.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Ahmed EK, Rogowska-Wrzesinska A, Roepstorff P, Bulteau AL, Friguet B. Protein modification and replicative senescence of WI-38 human embryonic fibroblasts. Aging Cell. 2010;9:252–272. doi: 10.1111/j.1474-9726.2010.00555.x. [DOI] [PubMed] [Google Scholar]
- Voghel G, Thorin-Trescases N, Farhat N, Mamarbachi AM, Villeneuve L, Fortier A, et al. Chronic treatment with N-acetyl-cystein delays cellular senescence in endothelial cells isolated from a subgroup of atherosclerotic patients. Mech Ageing Dev. 2008;129:261–270. doi: 10.1016/j.mad.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- Pizzimenti S, Menegatti E, Berardi D, Toaldo C, Pettazzoni P, Minelli R, et al. 4-hydroxynonenal, a lipid peroxidation product of dietary polyunsaturated fatty acids, has anticarcinogenic properties in colon carcinoma cell lines through the inhibition of telomerase activity. J Nutr Biochem. 2010;21:818–826. doi: 10.1016/j.jnutbio.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Neely MD, Zimmerman L, Picklo MJ, Ou JJ, Morales CR, Montine KS, et al. Congeners of N(alpha)-acetyl-L-cysteine but not aminoguanidine act as neuroprotectants from the lipid peroxidation product 4-hydroxy-2-nonenal. Free Radic Biol Med. 2000;29:1028–1036. doi: 10.1016/s0891-5849(00)00411-1. [DOI] [PubMed] [Google Scholar]
- Riahi Y, Kaiser N, Leibowitz G, Sima AV, Simionescu M, Sasson S.Mechanism of foam cell-induced endothelial cell senescence in atherosclerosis EAS Congress Abstract book80th European Atherosclerosis Society Congress, 2012, p 831.
- Hill BG, Haberzettl P, Ahmed Y, Srivastava S, Bhatnagar A. Unsaturated lipid peroxidation-derived aldehydes activate autophagy in vascular smooth-muscle cells. Biochem J. 2008;410:525–534. doi: 10.1042/BJ20071063. [DOI] [PubMed] [Google Scholar]
- Schutt F, Bergmann M, Holz FG, Kopitz J. Proteins modified by malondialdehyde, 4-hydroxynonenal, or advanced glycation end products in lipofuscin of human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2003;44:3663–3668. doi: 10.1167/iovs.03-0172. [DOI] [PubMed] [Google Scholar]
- Krohne TU, Stratmann NK, Kopitz J, Holz FG. Effects of lipid peroxidation products on lipofuscinogenesis and autophagy in human retinal pigment epithelial cells. Exp Eye Res. 2010;90:465–471. doi: 10.1016/j.exer.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Sparrow JR, Boulton M. RPE lipofuscin and its role in retinal pathobiology. Exp Eye Res. 2005;80:595–606. doi: 10.1016/j.exer.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Salabei JK, Cummins TD, Singh M, Jones SP, Bhatnagar A, Hill BG. PDGF-mediated autophagy regulates vascular smooth muscle cell phenotype and resistance to oxidative stress. Biochem J. 2013;451:375–388. doi: 10.1042/BJ20121344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi N, Slocum SL, Skoko JJ, Shin S, Kensler TW. When NRF2 talks, who's listening. Antioxid Redox Signal. 2010;13:1649–1663. doi: 10.1089/ars.2010.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, et al. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci USA. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levonen AL, Landar A, Ramachandran A, Ceaser EK, Dickinson DA, Zanoni G, et al. Cellular mechanisms of redox cell signalling: role of cysteine modification in controlling antioxidant defences in response to electrophilic lipid oxidation products. Biochem J. 2004;378 (Pt 2:373–382. doi: 10.1042/BJ20031049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S, Jaiswal AK, Forman HJ. The role of c-Jun phosphorylation in EpRE activation of phase II genes. Free Radic Biol Med. 2009;47:1172–1179. doi: 10.1016/j.freeradbiomed.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Vrishni S, Singh BK, Rahman I, Kakkar P. Nrf2-ARE stress response mechanism: a control point in oxidative stress-mediated dysfunctions and chronic inflammatory diseases. Free Radic Res. 2010;44:1267–1288. doi: 10.3109/10715762.2010.507670. [DOI] [PubMed] [Google Scholar]
- Siow RC, Ishii T, Mann GE. Modulation of antioxidant gene expression by 4-hydroxynonenal: atheroprotective role of the Nrf2/ARE transcription pathway. Redox Rep. 2007;12:11–15. doi: 10.1179/135100007X162167. [DOI] [PubMed] [Google Scholar]
- Sporn MB, Liby KT. NRF2 and cancer: the good, the bad and the importance of context. Nat Rev Cancer. 2012;12:564–571. doi: 10.1038/nrc3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi YC, Ansari GA, Awasthi S. Regulation of 4-hydroxynonenal mediated signaling by glutathione S-transferases. Methods Enzymol. 2005;401:379–407. doi: 10.1016/S0076-6879(05)01024-4. [DOI] [PubMed] [Google Scholar]
- Kong D, Kotraiah V. Modulation of aldehyde dehydrogenase activity affects (+/-)-4-hydroxy-2E-nonenal (HNE) toxicity and HNE-protein adduct levels in PC12 cells. J Mol Neurosci. 2012;47:595–603. doi: 10.1007/s12031-011-9688-y. [DOI] [PubMed] [Google Scholar]
- Murphy TC, Amarnath V, Gibson KM, Picklo MJ., Sr Oxidation of 4-hydroxy-2-nonenal by succinic semialdehyde dehydrogenase (ALDH5A) J Neurochem. 2003;86:298–305. doi: 10.1046/j.1471-4159.2003.01839.x. [DOI] [PubMed] [Google Scholar]
- Banaclocha MM. Therapeutic potential of N-acetylcysteine in age-related mitochondrial neurodegenerative diseases. Med Hypotheses. 2001;56:472–477. doi: 10.1054/mehy.2000.1194. [DOI] [PubMed] [Google Scholar]
- Circu ML, Aw TY. Glutathione and apoptosis. Free Radic Res. 2008;42:689–706. doi: 10.1080/10715760802317663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillancourt F, Fahmi H, Shi Q, Lavigne P, Ranger P, Fernandes JC, et al. 4-Hydroxynonenal induces apoptosis in human osteoarthritic chondrocytes: the protective role of glutathione-S-transferase. Arthritis Res Ther. 2008;10:R107. doi: 10.1186/ar2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher EP, Huisden CM, Gardner JL. Transfection of HepG2 cells with hGSTA4 provides protection against 4-hydroxynonenal-mediated oxidative injury. Toxicol In Vitro. 2007;21:1365–1372. doi: 10.1016/j.tiv.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Hinshelwood A, Gardner R, McGarvie G, Ellis EM. Mouse aldo-keto reductase AKR7A5 protects V79 cells against 4-hydroxynonenal-induced apoptosis. Toxicology. 2006;226:172–180. doi: 10.1016/j.tox.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Matsunaga T, Shinoda Y, Inoue Y, Endo S, El-Kabbani O, Hara A. Protective effect of rat aldo-keto reductase (AKR1C15) on endothelial cell damage elicited by 4-hydroxy-2-nonenal. Chem Biol Interact. 191:364–370. doi: 10.1016/j.cbi.2010.12.018. [DOI] [PubMed] [Google Scholar]
- Solito R, Chen CH, Mochly-Rosen D, Giachetti A, Ziche M, Donnini S. Mitochondrial aldehyde dehydrogenase-2 activation prevents beta amyloids induced endothelial cell dysfunction and restores angiogenesis. J Cell Sci. 2013;126 (Pt 9:1952–1961. doi: 10.1242/jcs.117184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappa A, Chen C, Koutalos Y, Townsend AJ, Vasiliou V. Aldh3a1 protects human corneal epithelial cells from ultraviolet- and 4-hydroxy-2-nonenal-induced oxidative damage. Free Radic Biol Med. 2003;34:1178–1189. doi: 10.1016/s0891-5849(03)00070-4. [DOI] [PubMed] [Google Scholar]
- Mitsuishi Y, Taguchi K, Kawatani Y, Shibata T, Nukiwa T, Aburatani H, et al. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell. 2012;22:66–79. doi: 10.1016/j.ccr.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Ruef J, Rao GN, Li F, Bode C, Patterson C, Bhatnagar A, et al. Induction of rat aortic smooth muscle cell growth by the lipid peroxidation product 4-hydroxy-2-nonenal. Circulation. 1998;97:1071–1078. doi: 10.1161/01.cir.97.11.1071. [DOI] [PubMed] [Google Scholar]
- Barrera G, Di Mauro C, Muraca R, Ferrero D, Cavalli G, Fazio VM, et al. Induction of differentiation in human HL-60 cells by 4-hydroxynonenal, a product of lipid peroxidation. Exp Cell Res. 1991;197:148–152. doi: 10.1016/0014-4827(91)90416-r. [DOI] [PubMed] [Google Scholar]
- Pizzimenti S, Laurora S, Briatore F, Ferretti C, Dianzani MU, Barrera G. Synergistic effect of 4-hydroxynonenal and PPAR ligands in controlling human leukemic cell growth and differentiation. Free Radic Biol Med. 2002;32:233–245. doi: 10.1016/s0891-5849(01)00798-5. [DOI] [PubMed] [Google Scholar]
- Zhang W, He Q, Chan LL, Zhou F, El Naghy M, Thompson EB, et al. Involvement of caspases in 4-hydroxy-alkenal-induced apoptosis in human leukemic cells. Free Radic Biol Med. 2001;30:699–706. doi: 10.1016/s0891-5849(01)00465-8. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Cornelius C, Mancuso C, Pennisi G, Calafato S, Bellia F, et al. Cellular stress response: a novel target for chemoprevention and nutritional neuroprotection in aging, neurodegenerative disorders and longevity. Neurochem Res. 2008;33:2444–2471. doi: 10.1007/s11064-008-9775-9. [DOI] [PubMed] [Google Scholar]
- Sykiotis GP, Bohmann D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev Cell. 2008;14:76–85. doi: 10.1016/j.devcel.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]