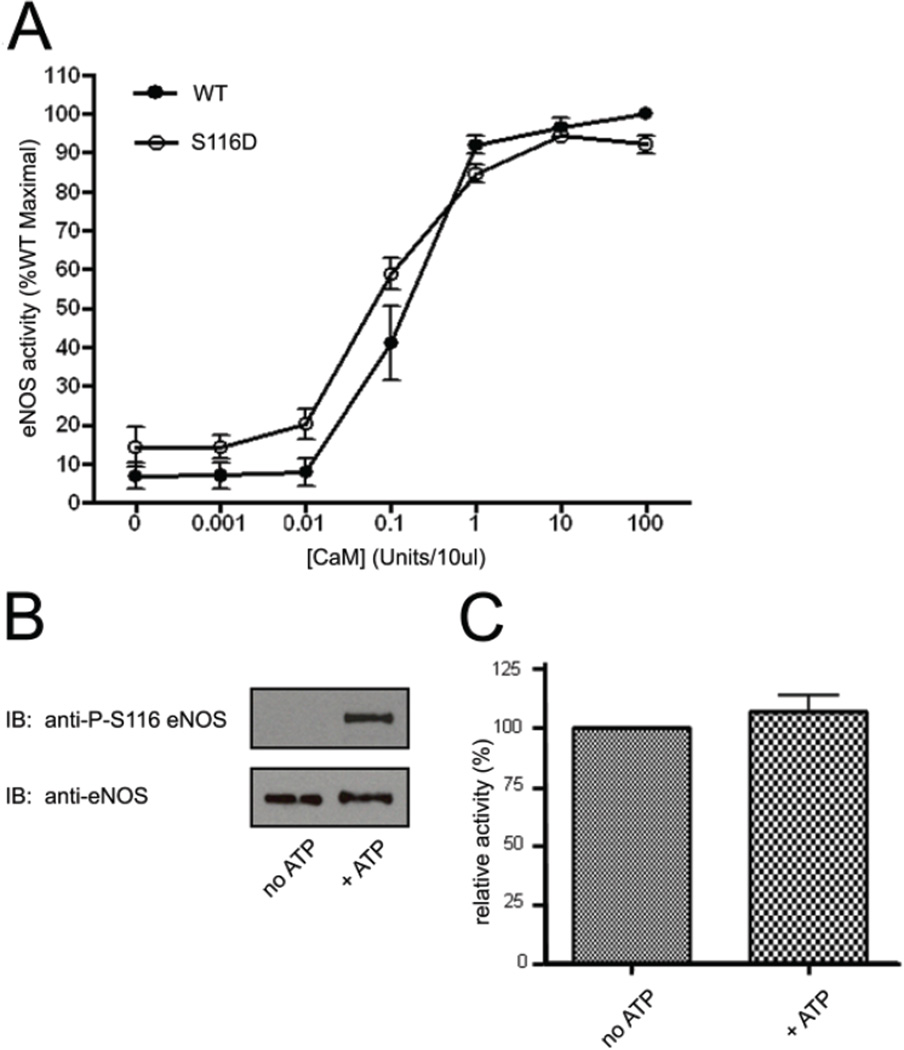
Fig. 3.
Effects of mimicking phosphorylation of eNOS at S116 or of ERK2-catalyzed phosphorylation of eNOS at S116 on the Ca2+-CaM-dependence and maximal activity of purified eNOS. A, wild-type (WT) and S116D forms of eNOS were expressed and purified from a baculovirus system. The relative specific activities of equal quantities of the two purified enzymes were then determined by arginine-to-citrulline conversion assay in the presence of excess cofactors and Ca2+ and in the presence of either no CaM or increasing concentrations of CaM. Results shown are expressed as percent of maximal activity of the wild-type enzyme from 3 separate, fresh (never frozen) preparations of each enzyme (means ± S.E.). B, equal quantities of purified WT eNOS were incubated in vitro with active ERK2 in the absence and presence of added ATP. S116 phosphorylation was determined by immunoblotting with anti-phospho-S116 eNOS antibody. C, relative activities of the non-phosphorylated (no ATP) and phosphorylated enzymes in the presence of excess Ca2+-CaM were determined by arginine-to-citrulline conversion assay. Results shown are means ± S.E. from measurements of 3 separate, fresh preparations of eNOS incubated with active ERK2 without and with ATP.