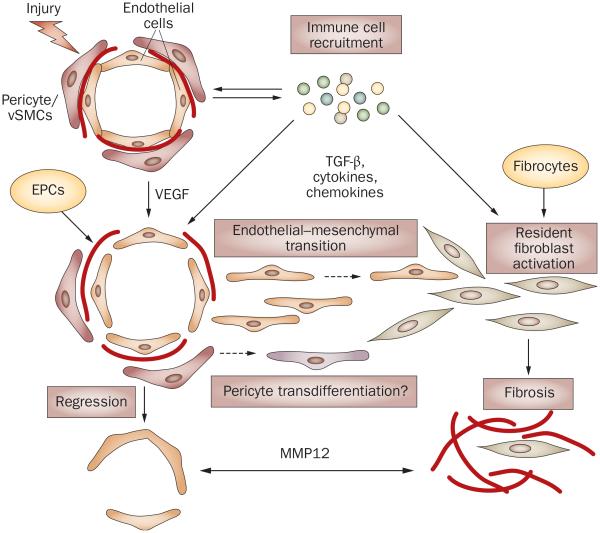
Figure 4.
Possible links between vasculopathy and fibrosis in SSc. Vascular injury due to an unknown trigger and the influx of the immune cells are the early events in SSc. High levels of VEGF and other proangiogenic mediators derived from the activated immune cells facilitate proliferation of endothelial cells and pericytes in an attempt to restore injured vessels. For reasons that are still not clearly understood, but which may involve imbalance of proangiogenic and antiangiogenic mediators, intrinsic properties of endothelial cells, and the fibroblast-secreted antiangiogenic factors (for example, MMP12) this process fails, leading to vessel regression. In the presence of TGF-β or other immune mediators (such as cytokines and chemokines), or both, endothelial cells could acquire a migratory phenotype through endothelial–mesenchymal transition and enter the surrounding tissue, where they further differentiate into collagen-producing cells. Likewise, pericytes could transdifferentiate into fibroblasts or myofibroblasts and produce collagen. In SSc, intrinsic abnormal properties of endothelial cells and pericytes might render vascular cells particularly susceptible to undergoing these transitions. Activated resident fibroblasts and fibrocytes that enter the injured tissue from the circulation are likely to represent additional source of collagen producing cells that contribute to fibrosis in SSc lesion. Abbreviations: EPCs, endothelial progenitor cells; VEGF, vascular endothelial growth factor; MMP12, matrix metalloproteinase 12; SSc, systemic sclerosis; TGF-β, transforming growth factor β.