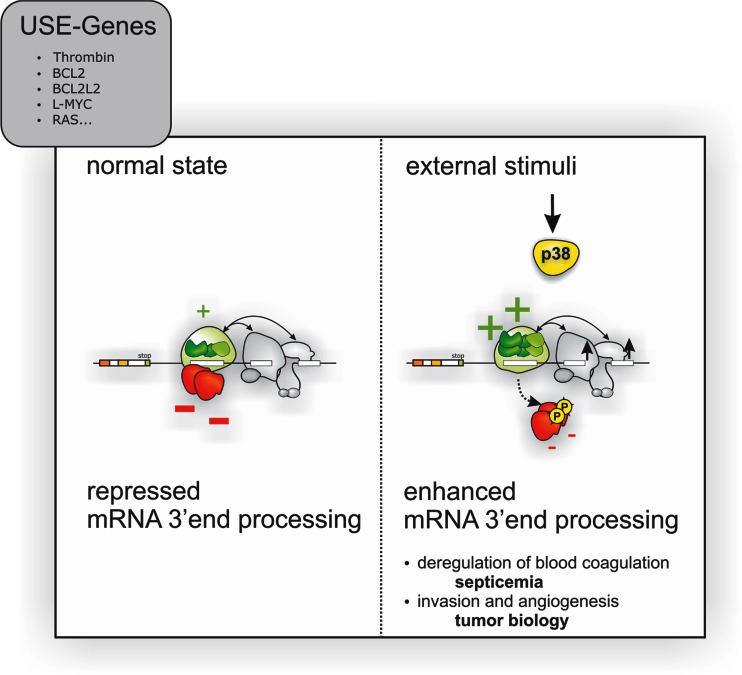
Fig. 5.
Extracellular stimuli induce thrombin gene expression by p38 MAPK activation. Extracellular stimuli activate p38 MAPK and thereby phosphorylate regulatory proteins (red), which “catalyzes” the remodeling of a stimulatory ribonucleoprotein (RNP) complexes (green) to up-modulate the efficiency of thrombin mRNA 3′ end processing. This mechanism allows modulating cellular functions, such as blood coagulation by controlling the amount of thrombin protein produced. Yet activation of this mechanism also appears to play an important role in other pathophysiological processes (such as tumorigenesis) and drives cellular programs involved in tumor invasion and neoangiogenesis by the activation of thrombin receptor signaling (F2R, PAR-1) and degradation of extracellular matrix (figure adopted from Cell Press [113])