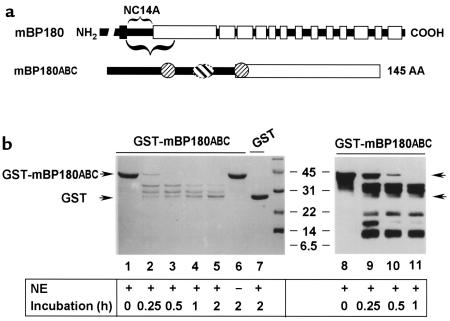
Figure 8.
In vitro degradation of mBP180ABC by neutrophil elastase. (a) The schematic diagram is a structural representation of mBP180. The vertical black bar designates the transmembrane domain. The COOH-terminal extracellular region is made up of 13 collagen triple-helical (open bar) and 14 noncollagenous (filled bar) domains. The purified recombinant GST-mBP180ABC fusion protein is 42 kDa, containing 26 kDa GST and a 145-amino acid fragment of the mBP180 extracellular domain. The mBP180ABC is made up of an 8-kDa noncollagenous NC14A (filled bar) and an 8-kDa collagenous region (open bar). One pathogenic (oval) and 2 nonpathogenic epitopes (circles) are located within the mBP180ABC (see ref. 51). (b) The GST-mBP180ABC fusion protein (lanes 1–6, 8–11) or GST alone (lane 7) was incubated with (lanes 1–5, 7–11) or without (lane 6) NE at 37°C for 0 minutes (lanes 1 and 8), 0.25 hours (lanes 2 and 9), 0.5 hours (lanes 3 and 10), 1 hour (lanes 4 and 11), or 2 hours (lanes 5–7). The products were then resolved by SDS-PAGE and detected by Coomassie blue staining (lanes 1–7) or immunoblot (lanes 8–11). Fragments of 34 kDa, 29 kDa, and 26 kDa were generated from the cleavage of the fusion protein by elastase (lanes 2–5 and 9–11). No degradation products were seen after incubation without NE (lane 6). GST was not degraded by NE (lane 7). Immunoblot using anti-mBP180ABC IgG identified 3 additional fragments with molecular weights of approximately 18 kDa, 15 kDa, and 9 kDa (lanes 9–11). The immunoreactivity of these bands with anti-mBP180ABC was abolished by preadsorption of the antibody with a 5-fold molar excess of GST-mBP180ABC fusion protein, but not with GST alone (data not shown).