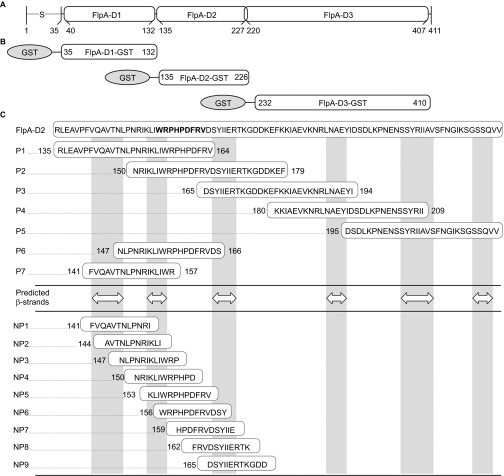
Figure 1.
FlpA domain organization, GST fusion proteins and synthetic peptides used in this study. (A) Schematic of FlpA structural features. FlpA harbors a signal peptide ‘S' at the amino terminus that is lipidated at A20 and three domains designated FlpA-D1, FlpA-D2 and FlpA-D3, homologous to Fn type three (FNIII) repeats. (B) N-terminally tagged GST fusion proteins used in this study. Each of the three FlpA FNIII-like repeats (FlpA-D1, FlpA-D2 and FlpA-D3) were expressed with GST tags. (C) Sequences of the synthetic peptides used in this study. Peptides are aligned relative to the FlpA-D2 sequence. The 30mer peptides P1–P5 overlap by 15 residues and span the entire FlpA-D2 sequence. Peptides P6 and P7 span predicted β-strands and flanking disordered regions of interest. White double arrowheads indicate the predicted β-strands and vertical gray boxes highlight their location (DOMpro 1.0). Peptides NP1–NP9 are 12mers that overlap by 9 residues. The sequence of the FlpA FBLM 156WRPHPDFRV164 is highlighted in bold.