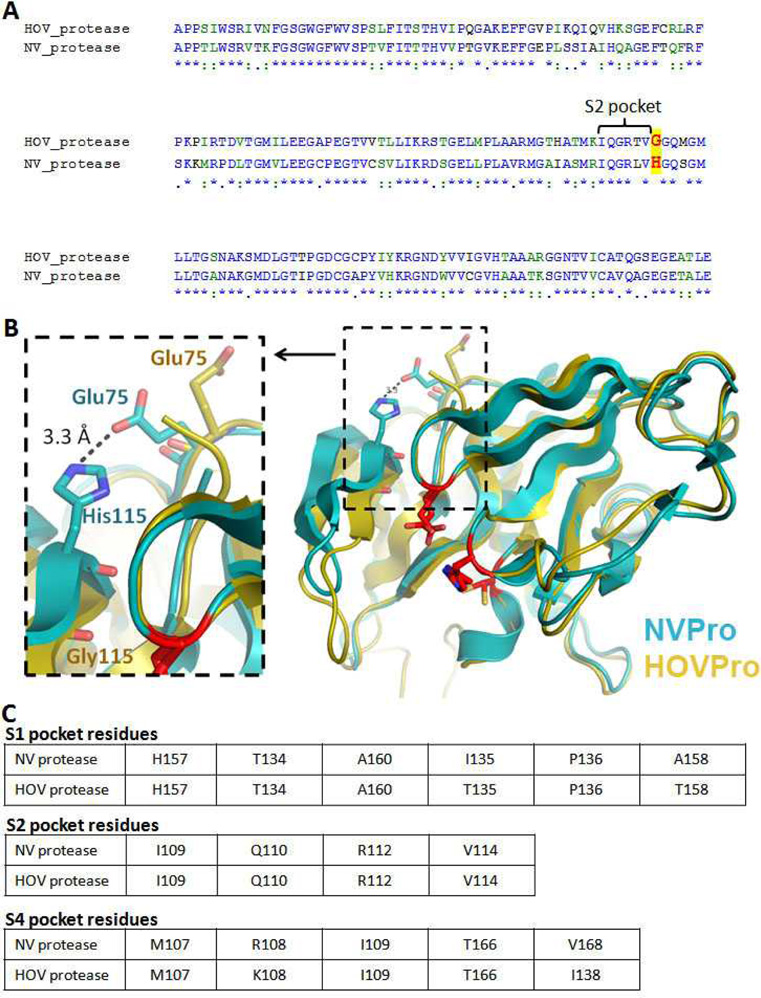
Fig. 2.
(A) Alignment of NVPro and HOVPro protein sequence. The conserved residues that compose the S2 pockets of the two proteins are labeled with a bracket and a major difference is highlighted red letters; (B) Superposition of the crystal structure of NVPro (PDB: 2FYQ) shown in cyan and a homology model of HOVPro structure in gold. The S2 pocket loop (bII-cII) shows a different conformation in the HOV protease, which appears to be due to the Gly115 residue. In the NV protease the residue at the 115 position is His; (C) Tables depict the conserved residues that compose the S1, S2 and S4 pockets, in NV and HOV proteases.