Table 1.
Reaction Optimization for Conversion of Tetralone Pivaloyl Oxime to 1-Aminonaphthalene (2)a
 | ||||
|---|---|---|---|---|
| Entry | palladium | ligand | additive | % conv/ % yieldb |
| 1 | 10% Pd(P(o-tol)3)2 | 50/ 24 | ||
| 2 | 5% Pd2(dba)3 | 56/ 23 | ||
| 3 | 10% Pd(PPh3)4 | 96/ 70 | ||
| 4 | 5% Pd2(dba)3 | 20% PPh3 | 99/ 63 | |
| 5 | 5% Pd2(dba)3 | 20% PCy3 | 99/ 68 | |
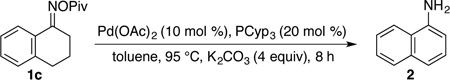
| 6 | 10% Pd(OAc)2 | 20% PCyp3 | 97/ 82 | |
| 7 | 10% Pd(OAc)2 | 20% PCy3 | 97/ 80 | |
| 8 | 10% Pd(OAc)2 | 20% PPh3 | 74/ 37 | |
| 9 | 10% Pd(OAc)2 | 20% dppe | 73/ 62 | |
| 10 | 10% Pd(OAc)2 | 20% dppf | 78/ 44 | |
| 11 | 10% Pd(OAc)2 | 20% PCyp3 | 30% PivOH | 99/ 87 |
Reaction condition of Entry 11: 1c (0.2 mmol), Pd(OAc)2 (0.02 mmol), tricyclopentylphosphine (0.04 mmol), PivOH (0.06 mmol), K2CO3 (0.8 mmol), toluene (2.2 mL).
1H NMR yield; internal standard = tetrachloroethane.
