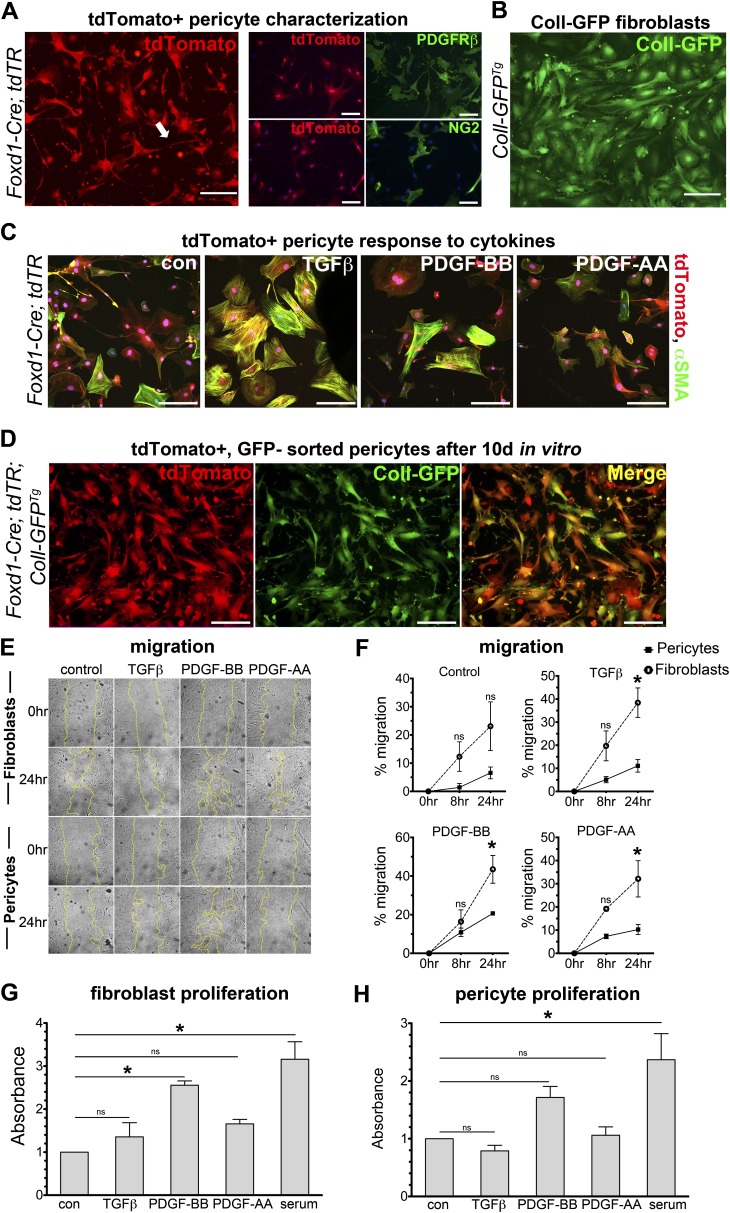
Figure 5.
Foxd1 progenitor–derived pericytes and Coll-GFP+ resident fibroblasts exhibit morphologic and behavioral differences in two-dimensional culture. (A) Fluorescence images of primary cultures of the Foxd1 progenitor–derived cells (pericytes) permanently expressing tdTomato, isolated from Foxd1-Cre; tdTR mice (left). Foxd1 progenitor–derived cells exhibit long foot processes characteristic of pericytes in vitro (arrow). Immunostaining of tdTomato+ cells in vitro demonstrates colabeling of PDGFRβ in tdTomato+ cells, whereas only some tdTomato+ cells colabel with NG2 (right). (B) Coll-GFP+ fibroblasts from Coll-GFPTg mice at passage 2 show a distinctly different morphology from Foxd1 progenitor–derived pericytes. (C) tdTomato+ pericytes isolated from Foxd1-Cre;tdTR mice activate αSMA (green) protein expression and stress fiber formation in response to TGFβ stimulation in vitro after 24 hours. (D) Foxd1 progenitor–derived pericytes (tdTomato+, Coll-GFP−) isolated from Foxd1-Cre; tdTR; Coll-GFPTg mice shown after two passages in culture. Foxd1-derived pericytes show persistent expression of red fate marker (left, red) in vitro. Nearly all Foxd1 progenitor–derived pericytes activate high levels of Coll-GFP (middle, green) in vitro (bars = 50 μm). (E and F) Coll-GFP+ fibroblasts show increased migration compared with pericytes in response to TGFβ (1 ng/ml), PDGF-BB (50 ng/ml), and PDGF-AA (30 ng/ml) at 24 hours control: serum free (mean ± SEM, n = 3, *P < 0.05 fibroblast vs. pericyte). (G and H) PDGF-BB treatment increased proliferation in Coll-GFP+ fibroblasts compared with control (serum-free) at 72 hours, but not in pericytes. Proliferation measured by BrdU ELISA reported as absorbance relative to control. (mean ± SEM, n = 3, *P < 0.05 vs. control).