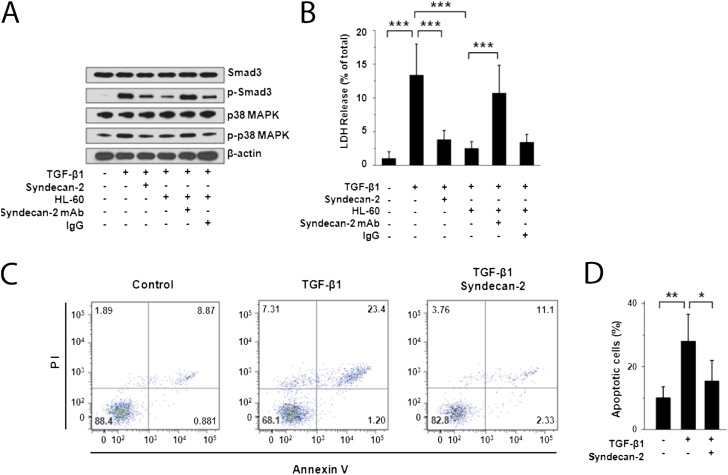
Figure 4.
Inhibition of cell death/apoptosis and transforming growth factor (TGF)-β1 signaling by recombinant syndecan-2 in small airway epithelial cells (SAEC). (A) Western immunoblot analyses of primary human SAEC stimulated with TGF-β1 (5 ng/ml) for 2 hours and incubated with recombinant human syndecan-2 demonstrated reduced phosphorylation of SMAD3 and p38 MAPK compared with SAEC that were not incubated with syndecan-2. Phosphorylation of SMAD3 and p38 MAPK was also reduced in SAEC stimulated with TGF-β1 and cocultured with HL-60 monocytic cells, which secrete syndecan-2. This inhibitory effect on SMAD3 and p38 MAPK phosphorylation was lost when these cells were incubated with anti–syndecan-2 antibody but not with an IgG control antibody. (B) Cell death measured by lactate dehydrogenase (LDH) release of SAEC stimulated with TGF-β1 (5 ng/ml) for 48 hours and incubated with recombinant syndecan-2 or HL-60 cells was significantly reduced compared with cells incubated without syndecan-2 or HL-60 cells (***P < 0.001). This inhibitory effect on cell death was reversed when these cells were incubated with anti–syndecan-2 antibody, but not with an IgG control antibody. LDH release assays are representative of at least three independent experiments performed in triplicate. (C) SAEC stimulated with TGF-β1 (5 ng/ml) were coincubated with or without recombinant syndecan-2 for 48 hours and stained with Annexin V-FITC/PI. Flow cytometry showed live cells in the lower left quadrant, early apoptotic cells in the lower right quadrant, late apoptotic cells in the upper right quadrant, and necrotic cells in the upper left quadrant. Data shown were representative of three independent experiments. (D) Percentage of apoptotic SAEC stimulated with TGF-β1 alone (28.1 ± 8.5%) was significantly higher than that of TGF-β1–stimulated cells treated with recombinant syndecan-2 (15.4 ± 6.5%; *P = 0.03) or unstimulated SAEC (10.2 ± 4.2%; **P = 0.009).