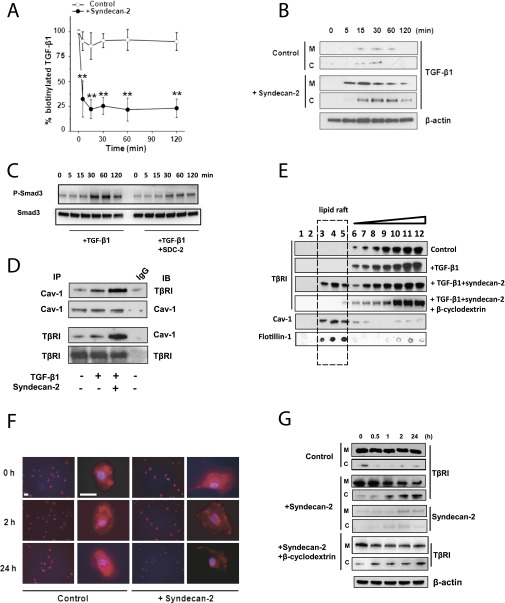
Figure 5.
Syndecan-2 enhances transforming growth factor (TGF) β receptor (TβRI) and ligand’s internalization via caveolae pathway. (A) Small airway epithelial cells (SAEC) were cultured with and without syndecan-2 (Control); biotinylated TGF-β1 was added to the medium and measured at different time points. Approximately 25% of total labeled TGF-β1 remained in the medium after 15 minutes’ incubation with syndecan-2 compared with approximately 80% in control. (B) SAEC lysates of cells incubated with and without syndecan-2 were separated into membrane (M) and cytosolic (C) fractions, and biotinylated TGF-β1 was precipitated by Avidin beads. Immunoblots using anti–TGF-β1 antibody showed internalization of TGF-β1 after 15 minutes of incubation with syndecan-2. (C) SAEC were treated with TGF-β1 alone or TGF-β1 with syndecan-2 for 0 to 120 minutes, then phosphorylation of SMAD3 in cell lysates was measured by immunoblotting. (D) Coimmunoprecipitation showed increased interaction between Caveolin-1 (Cav-1) and TβRI when SAEC were incubated with recombinant syndecan-2 and TGF-β1 compared with cells incubated with TGF-β1 alone. (E) SAEC (Control) or SAEC incubated with TGF-β1 with or without syndecan-2 were solubilized, and the lipid raft/caveolae fraction was separated by sucrose gradient centrifugation. Western immunoblot analyses of TβRI, caveolin-1 (Cav-1), and flotillin-1 in 12 cell fractions from SAEC showed that syndecan-2 incubation caused a shift of TβRI from non–lipid raft fractions to the lipid raft fractions. Adding 10 mM β-cyclodextrin to disrupt lipid raft–caveolin internalization inhibited the shift of TβRI induced by syndecan-2. (F) SAEC incubated with or without syndecan-2 were stimulated with 10 ng/ml of TGF-β1 for 0, 2, or 24 hours. Cells were stained with anti-TβRI antibody (red) and DAPI (blue). Immunostaining of TβRI in SAEC was decreased after 2 or 24 hours in the presence of syndecan-2 (scale bar = 10 µm). (G) Immunoblot using anti-TβR1 antibody is shown of membrane (M) and cytosolic (C) fractions of SAEC stimulated with TGF-β1 with or without syndecan-2 (Control) for 0, 0.5, 1, 2, and 24 hours. TβRI in SAEC was expressed predominantly in the membrane (M) fraction. However, TβRI internalized from the membrane to cytosolic fraction in SAEC incubated for 1 hour with syndecan-2. Recombinant syndecan-2 was traced by biotinylation. In both membrane and cytosolic fractions, biotinylated syndecan-2 was precipitated by Avidin beads and immunoblotted with syndecan-2 antibody. The blot shows that syndecan-2 was also internalized from the membrane to the cytosolic fraction. The addition of 10 mM β-cyclodextrin inhibited the internalization of TβRI induced by syndecan-2.