Figure 1.
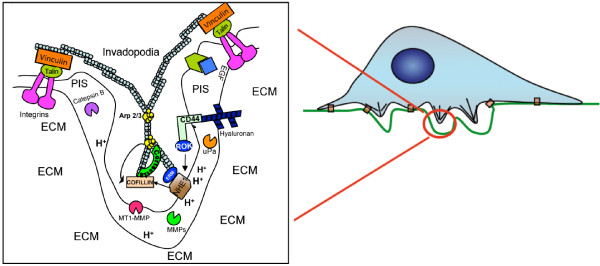
Model of localization and role of NHE1 in invadopodia. The insert is a magnification of the F-actin-enriched cellular protrusions into the ECM that are responsible for ECM degradation and are known as invadopodia. Invadopodia formation is activated by integrin binding to the ECM and their activity further increased through the CD44 (activated by its ligand Hyaluronan) and EGFR receptors located in the membrane. The integrin receptors are connected to the cytoskeleton (blue circles) through the proteins Talin and Vinculin. The proteases cathepsin B, D and L, Urokinase Plasminogen Activator and the matrix metalloproteinases MMP-2 and MMP-9 are released extracellularly while the MT1-MMP is localized within the membrane and participates together with Cathepsin B in the processing of inactive pro-MMP-2 into active MMP-2. Glycolytic enzymes are enriched in invadopodia, leading to the localized production of protons. These protons are secreted via an active NHE1 that is recruited to the invadopodia through integrin binding and further stimulated by CD44 and EGFR. NHE1 with its two functions (scaffolding protein and ion exchanger) leads to membrane protrusion and proteolysis. As a proton transporter, NHE1 promotes invasion through its control of the acidification of the peri-invadopodial space where NHE1 proton secreting activity and proteases act in concert to degrade the ECM during invasion. Further, the NHE1-dependent alkalinization of the invadopodia cytosol results in a phosphorylation of cortactin with the subsequent release of cofilin which promotes actin polymerization, growth of the invadopodia cytoskeleton and invadopodia protrusion. Secondly, NHE1 also promotes invadopodial formation via its interaction with the cytoskeleton through its binding to the actin anchoring protein, ezrin, which, reciprocally is responsible for the localization of NHE1 to the invadopodia in response to ECM and growth factor receptor activation. PIS: PeriInvadopodia Space; ECM: ExtraCellular Matrix. Please see text for discussion and references.
